Liposomal oxymatrine in hepatic fibrosis treatment: formulation, in vitro and in vivo assessment
- PMID: 24515270
- PMCID: PMC4037472
- DOI: 10.1208/s12249-014-0086-y
Liposomal oxymatrine in hepatic fibrosis treatment: formulation, in vitro and in vivo assessment
Abstract
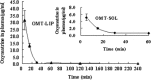
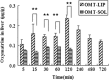
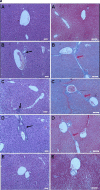
The aim was to develop a liposomal oxymatrine conjugating D-alpha tocopheryl polyethylene glycol 1000 succinate (OMT-LIP) for enhanced therapeutics of hepatic fibrosis. OMT-LIP was prepared using the remote loading method. The influences of formulation compositions on the encapsulation efficiency of OMT-LIP were investigated. Mean particle size, zeta potential, morphology, in vitro release, fibrotic liver targeting, and therapeutics of OMT-LIP were thoroughly assessed. The intraliposomal buffer composition and concentration, extraliposomal phase composition and pH, types of phospholipid, lipid molar ratio composition, and theoretical drug loading are crucial factors to entrap OMT into liposomes. The optimum OMT-LIP presented spherically unilamellar microstructures with entrapment efficiency of 79.7 ± 3.9%, mean particle size of 121.6 ± 52.9 nm, and zeta potential of -5.87 mV. OMT-LIP significantly increased the accumulation of OMT in the fibrotic liver with an 11.5-fold greater AUC than OMT solution in the dimethylnitrosamine (DMN)-induced hepatic fibrosis animals. OMT-LIP could be a potential strategy to improve treatment outcomes for hepatic fibrosis, showing the protective effects to mice given CCl4 and the enhanced therapeutics to mice with either DMN or CCl4-induced hepatic fibrosis.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

