A bidirectional antagonism between aPKC and Yurt regulates epithelial cell polarity
- PMID: 24515345
- PMCID: PMC3926957
- DOI: 10.1083/jcb.201308032
A bidirectional antagonism between aPKC and Yurt regulates epithelial cell polarity
Abstract
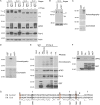
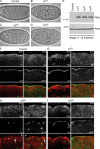
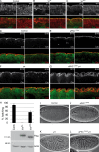
During epithelial cell polarization, Yurt (Yrt) is initially confined to the lateral membrane and supports the stability of this membrane domain by repressing the Crumbs-containing apical machinery. At late stages of embryogenesis, the apical recruitment of Yrt restricts the size of the apical membrane. However, the molecular basis sustaining the spatiotemporal dynamics of Yrt remains undefined. In this paper, we report that atypical protein kinase C (aPKC) phosphorylates Yrt to prevent its premature apical localization. A nonphosphorylatable version of Yrt dominantly dismantles the apical domain, showing that its aPKC-mediated exclusion is crucial for epithelial cell polarity. In return, Yrt counteracts aPKC functions to prevent apicalization of the plasma membrane. The ability of Yrt to bind and restrain aPKC signaling is central for its role in polarity, as removal of the aPKC binding site neutralizes Yrt activity. Thus, Yrt and aPKC are involved in a reciprocal antagonistic regulatory loop that contributes to segregation of distinct and mutually exclusive membrane domains in epithelial cells.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

