Diacerein for osteoarthritis
- PMID: 24515444
- PMCID: PMC10712695
- DOI: 10.1002/14651858.CD005117.pub3
Diacerein for osteoarthritis
Abstract
Background: Osteoarthritis (OA) is one of the most prevalent musculoskeletal diseases. There is currently no consensus on what is the best treatment to improve OA symptoms and slow disease progression. Diacerein is an anthraquinone synthesised in 1980 that interferes with interleukin-1, an inflammatory mediator. It has been proposed that diacerein acts as a slow-acting, symptom-modifying and perhaps disease-structure-modifying drug for OA. This is an update of a Cochrane review first published in 2006.
Objectives: To assess the benefits and harms of diacerein for the treatment of adults with OA when compared with placebo and other pharmacologically active interventions (nonsteroidal anti-inflammatory drugs (NSAIDs) and other symptom-modifying, slow-acting drugs) for OA.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) - The Cochrane Library, Issue 10, 2013, MEDLINE (1966 to 2013), EMBASE (1980 to 2013), LILACS (1982 to 2013), and ACP Journal Club, and we handsearched reference lists of published articles. We also searched the World Health Organization International Clinical Trials Platform ( http://www.who.int/trialsearch/Default.aspx) to identify ongoing trials and screened reference lists of retrieved review articles and trials to identify potentially relevant studies. All searches were up to date as of March 2013. Pharmaceutical companies and authors of published articles were contacted. We searched the websites of the regulatory agencies using the keyword 'diacerein' in November 2013. No language restrictions were applied.
Selection criteria: Studies were included if they were randomised or quasi-randomised controlled trials that compared diacerein with placebo or another active pharmacological intervention in participants with OA.
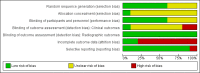
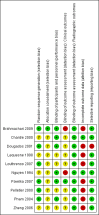
Data collection and analysis: Data abstraction and quality assessment were performed by two independent investigators, and their results were compared. The Cochrane risk of bias tool was used. The quality of evidence obtained was assessed using the GRADE approach.
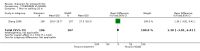
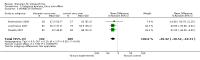
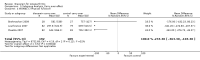
Main results: We identified three new trials (141 participants), and this updated review now includes 10 trials, totalling 2,210 participants. The most frequent risk of bias was incomplete outcome data, identified in approximately 80% of the studies. Allocation concealment and random sequence generation were unclear in 90% and 40% of the studies, respectively, because of poor reporting.Low-quality evidence from six trials (1,283 participants) indicates that diacerein has a small beneficial effect on overall pain (measured on a 100 mm visual analogue scale) at three to 36 months (mean difference (MD) -8.65, 95% confidence interval (CI) -15.62 to -1.68), which is equivalent to a 9% pain reduction in the diacerein group (95% CI -16% to -2%) compared with the placebo group. This benefit may not be clinically significant.No statistically significant differences in physical function (4 studies, 1006 participants) were noted between the diacerein and placebo groups (Lequesne impairment index, 0 to 24 points) (MD -0.29, 95% CI -0.87 to 0.28).Low-quality evidence from two trials (616 participants) on slowing of joint space narrowing (a decrease greater than 0.50 mm) in the knee or hip favoured diacerein over placebo (risk ratio (RR) 0.85, 95% CI 0.72 to 0.99), with an absolute risk difference of -6% (95% CI -15% to 2%) and a number needed to treat for an additional beneficial outcome (NNTB) of 14 (95% CI 8 to 203). Analysis of the knee joint alone (1 study, 170 participants) did not reach statistical significance (RR 0.94, 95% CI 0.51 to 1.74).None of the trials of diacerein versus placebo measured quality of life. According to one trial (161 participants), which compared diacerein versus non-steroidal anti-inflammatory drugs (NSAIDs), the quality of life of participants in the two groups (as assessed by the Short Form (SF)-36 health survey questionnaire (0 to 800 sum score)) did not differ significantly (MD -40.70, 95% CI -85.20 to 3.80).Low-quality evidence from seven trials showed significantly more adverse events in the diacerein group compared with the placebo group after two to 36 months, mainly diarrhoea (RR 3.52, 95% CI 2.42 to 5.11), with an absolute risk increase of 24% (95% CI 12% to 35%), and a number needed to treat for an additional harmful outcome (NNTH) of 4 (95% CI 3 to 7).No statistically significant differences in participant withdrawal due to adverse events were seen at two to 36 months for diacerein compared with placebo (RR 1.29, 95% CI 0.83 to 2.01).A search of regulatory websites found a recommendation from the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) that the marketing authorization of diacerein should be suspended across Europe because of harms (particularly the risk of severe diarrhoea and potentially harmful effects on the liver) outweighing benefits. However, this guidance is not final as the PRAC recommendation will be re-examined.
Authors' conclusions: In this update, the strength of evidence for effectiveness outcomes was low to moderate. We confirmed that symptomatic benefit provided by diacerein in terms of pain reduction is minimal. The small benefit derived in terms of joint space narrowing is of questionable clinical relevance and was observed only for OA of the hip. With respect to adverse effects of diacerein, diarrhoea was most frequent. Given the recent guidance issued by the EMA recommending suspension of diacerein in Europe, the EMA website should be consulted for further recommendations regarding the use of diacerein.
Conflict of interest statement
None known.
Figures
























Update of
-
Diacerein for osteoarthritis.Cochrane Database Syst Rev. 2006 Jan 25;(1):CD005117. doi: 10.1002/14651858.CD005117.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 Feb 10;(2):CD005117. doi: 10.1002/14651858.CD005117.pub3. PMID: 16437519 Updated.
References
References to studies included in this review
Brahmachari 2009 {published data only}
-
- Brahmachari B, Chatterjee S, Ghosh A. Efficacy and safety of diacerein in early knee osteoarthritis: a randomized placebo‐controlled trial. Clinical Rheumatology 2009;28:1193‐1198. - PubMed
Chantre 2000 {published data only}
-
- Chantre P, Cappelaere A, Leblan D, Guedon D, Vandermander J, Fournie B. Efficacy and Tolerance of Harpagophytum Procumbens versus Diacerhein in Treatment of Osteoarthritis. Phytomedicine 2000;7:177‐183. - PubMed
Dougados 2001 {published data only}
-
- Dougados M, Nguyen M, Berdah L, Maziéres B, Vignon E, Lequesne M. Evaluation of Structure‐Modifying Effects of Diacerein in Hip Osteoarthritis. Arthritis & Rheumatism 2001;44(11):2539‐2547. - PubMed
Lequesne 1998 {published data only}
-
- Lequesne M, Berdah L, Gérentes I. Efficacy and Safety of Diacerein for the treatment of Knee and Hip Osteoarthritis [Efficacité et tolérance de la diacerhéine dans le traitment de la gonarthrose et de la coxarthrose]. La Revue du Praticien 1998;48:S31‐S35. - PubMed
Louthrenoo 2007 {published data only}
-
- Louthrenoo W, Nilganuwong S, Aksaranugraha S, Asavatanabodee P, Saengnipanthkul S. The efficacy, safety and carry‐over effect of diacerein in the treatment of painful knee osteoarthitis: a randomised, double‐blind, NSAID‐controlled study. Osteoarthritis and Cartilage 2007;15:605‐614. - PubMed
Nguyen 1994 {published data only}
-
- Ngyen M, Dougados M, Berdah L, Amor B. Diacerhein in The Treatment of Osteoarthritis of The Hip. Arthritis & Rheumatism 1994;37(4):529‐36. - PubMed
Pavelka 2007 {published data only}
-
- Pavelka K, Trc T, Karpas K, Vítek P, Sedlacková M, Vlasáková V, Böhmová J, Rovenský J. The Efficacy and Safety of Diacerein in the Treatment of Painful Osteoarthritis of the Knee. Arthritis & Rheumatism 2007;56:4055‐4064. - PubMed
Pelletier 2000 {published data only}
-
- Pelletier JP, Yaron M, Haraoui B, Cohen P, Nahir M A, Choquette D, et al. Efficacy and Safety of Diacerein in Osteoarthritis of the Knee. Arthritis and Rheumatism 2000;43(10):2339‐2348. - PubMed
Pham 2004 {published data only}
-
- Pham T, Henanff AL, Ravaud P, Dieppe P, et al. Evaluation of symptomatic and structural efficacy of a new hyaluronic acid (HA) compound, (NRD101), when compared to diacerein and placebo in one‐year randomized controlled study in symptomatic knee osteoarthritis. Annals of Rheumatic Diseases 2004;63(12):161‐7. - PMC - PubMed
Zheng 2006 {published data only}
-
- Zheng Wen‐Jie, Tang Fu‐Lin, Li Jun, Zhang Feng‐Chun, Li Zhan‐Guo, Su Yin, Wu Dong‐Hai, Ma Li, Zhou Hui‐Qiong, Huang Feng, Zhang Jiang‐Lin, Liang Dong‐Feng, Zhou Yi‐Xiong, Xu Hui. Efficacy and safety of diacerein in osteoarthritis of the knee: a randomized, multicenter, double‐dummy, diclofenac‐controlled trial in China. APLAR Journal of Rheumatology. 2006; Vol. 9:64‐69.
References to studies excluded from this review
Adami 1985 {published data only}
-
- Adami S, Bortolotti R, Guarrera G, Marini G, Rosini S, Zampieri A, Lo Cascio V. Diacerein in the Treatment of Degenerative Arthropathy [La Diacetilreina nel Trattamento delle Artropatie Degenerative]. La Clinica Terapeutica 1985;112:439‐443. - PubMed
Ascherl 1994 {published and unpublished data}
-
- Ascherl R. Double blind, placebo‐controlled multicentre, phase III study of the efficacy and tolerability of diacerein (DA39) in patients with osteoarthritis of the knee (University of Lubeck). Köln, Germany:Madaus AG;. unpublished final clinical study report October 10, 1994. Madaus Report DA39KO.13.
Baliga 2010 {published data only}
-
- Baliga VP, Bolmall CS, Jagiasi JD, Kumar MSA, Sankaralingam K, Veerappan V. Efficacy, safety and tolerability of diacerein MR 100 mg versus diacerein 50 mg in adult patients with osteoarthritis of the knee. Osteoarthritis and Cartilage 2010;18 SUPPL 2:S252.
Bogliola 1991 {published data only}
-
- Bogliolo A, Loi A, Perpignano G. "Fangobalneotherapy" and Diacerein in the Treatment of Hip and Knee Osteaoarthrosis [Fangobalneoterapia e Diacereina nel Trattamento dell'Osteoartrosi dell'Anca e del Ginocchio]. La Clinica Terapeutica 1991;137:3‐8. - PubMed
Carrabba 1987 {published data only}
-
- Carrabba M, Mele G, Chevallard M, Angelini M. Diacereine, an "original"approach to the treatment of degenerative and/or extra‐articular rheumatism. [Diacereina: un approccio "originale" nel trattamento dei reumatismi degenerativi e/o extra‐articolari]. Minerva Medica 1987;78:179‐185. - PubMed
Delcambre 1994 {published data only}
-
- Delcambre B, Taccoen A. ART 50 Study in the Update Rheumatologic Practice [Étude D'ART 50 en Pratique Rhumatologique Courante]. Revue Du Rhumatisme 1994;61:142S‐146S. - PubMed
Delcambre 1996 {published data only}
-
- Delchambre B, Taccoen A. Study of ART 50 in the rheumatologic daily practice [Étude d'ART 50 en pratique rhumatologique quotidienne]. Le Revue du Praticien 1996;46:S49‐S52. - PubMed
Fagnani 1998 {published data only}
-
- Fagnani F, Bouvenot G, Valat J‐P, Bardin T, Berdah L, Lafuma A, Bono I, Escchwege E, Dreiser R‐L. Medico‐Economic Analysis of Diacerein With or Without Standard Therapy in the Treatment of Osteoarthritis. Pharmacoeconomics 1998;13:135‐146. - PubMed
Fioravanti 1985 {published data only}
-
- Fioravanti A, Marcolongo R. Therapeutic effectiveness of diacerhein (DAR) in arthrosis of the knee and hip. Report presented at: The Toscana Medicina Symposuim on Diacereina. October 1, 1985.
Kay 1980 {published data only}
-
- Kay AGL, Griffiths LG, Volans GN, Grahame R. Prelimirary experience with diacetylrhein in the treatment of osteoarthritis. Current Medical Research and Opinion 1980;6(8):548‐551. - PubMed
Leblan 2000 {published data only}
-
- Leblan D, Chantre P, Fournié B. Harpagophytum procumbens in the treatment of knee and hip osteoarthritis. Four‐month results of a prospective, multicenter, double‐blind trial versus diacerhein. Join Bone Spine 2000;67:462‐7. - PubMed
Linguetti 1982 {published data only}
-
- Linguetti M, D'Ambrosio PL, Grezia F, Sorrentino P, Lingetti E. A Controlled Study in the Treatment of Osteoarthritis with Diacetylrhein (ARTRODAR). Current Therapeutic Research 1982;31(3):408‐412.
Mantia 1987 {published and unpublished data}
-
- Mantia C. A controlled study of the efficacy and tolerability of diacetylrhein in the functional manifestations of osteoarthritis of the hip and the knee: a double‐blind study versus diclofenac. Palermo Hospital, Palermo, Italy; 1987.
Marcolongo 1988 {published data only}
-
- Marcolongo R, Fioravanti A, Adami S, Tozzi E, Mian M, Zampieri A. Efficacy and Tolerability of Diacerhein in the Treatment of Osteoarthrosis. Current Therapeutic Research 1988;43(5):878‐887.
Mathieu 1999 {published data only}
-
- Mathieu P. Interleucine 1 ‐ Results of a pilot study with diacerein (ART 50) for Knee Osteoarthrosis [L'interleukine 1 ‐ Résultats d'une étude "pilote" avec la diacerhéine (ART 50) dans la gonarthrose]. La Revue du Praticien 1999;49:S15‐S18. - PubMed
Mattara 1985 {published data only}
-
- Mattara L. [DAR: indagini "controllate" nel trattamento della osteoartosi ]. Relazione presentata al LXXXVI Congresso Naz. Società Ital. Medicina Interna ‐ Sorrento. 1985; Vol. S:59.
Mazzaro 1989 {published data only}
-
- Mazzaro C, Bocchieri E, Tesolin FG, Ventre L, Romagnoli A. Clinical Evaluation of Diacereine in Osteoarthrosis Treatment [Valutazione clinica della Diacereina nel Trattamento dell'osteoartrosi]. Minerva Medica 1989;80(9):1025‐1027. - PubMed
Mordini 1986 {published and unpublished data}
-
- Mordini M, Nencioni C, Lavagni A, Camarri E. Diacerhein vs naproxen in coxo‐gonarthrosis: double‐blind randomized study. Abstract presented at: The 27th Congress of the Italian Society of Rheumatology. October 30‐November 2, 1986.
Pietrogrande 1985 {published data only}
-
- Pietrogrande V, Leonardi M, Pacchioni C. Results of a clinical trial with a new drug, diacerhein in arthrosic patiens.. Report presented at: The LXXXVI Congress of the Italian National Society of Internal Medicine;. September 24, 1985.
Portioli 1987 {published and unpublished data}
-
- Portioli IA. Naproxen‐controlled study on efficacy and tolerability of diacetylrhein in the functional manifestations of osteoarthritis of the knee and hip: a double blind study versus naproxen. unpublished clinical study report; Santa Maria Nuova Hospital, Reggio Emilia, Italy 1987.
Renapurkar 2010 {published data only}
-
- Renapurkar DK. Mathur S, Rao KLJ. Evaluation of efficacy and safety of diacerein in osteoarthritis of knee joint. International Journal of Pharma and Bio Sciences 2010;1(3):1‐11.
Schulitz 1994 {published and unpublished data}
-
- Schulitz KP. Clinical investigation of the efficacy and tolerance of diacetylrhein (DAR) in the treatment of osteoarthritis of the knee. Köln, Germany: Madaus AG. Unpublished final clinical study report. Madaus Report RDA139. October 27 1994.
Seisenbayev 2012 {published data only}
-
- Seisenbayev A, Togizbaev. Three component treatment of osteoarthiitis. Rheumatology 2012;51 SUPPL 1:i29‐i30.
Sharma 2008 {published data only}
-
- Sharma A, Rathod R, Baliga VP. An Open Prospective Study on Postmarketing Evaluation of the Efficacy and Tolerability of Diacerein in Osteo‐arthritis of the knee (DOK). Journal of Indian Medical Association 2008;106:31‐34. - PubMed
Singh 2012 {published data only}
-
- Singh K, Sharma R, Rai J. Diacerein as adjuvant to diclofenac sodium in osteoarthritis knee. International journal of rheumatic diseases 2012;15(1):69‐77. [PUBMED: 22324949] - PubMed
Tang 2004 {published data only}
-
- Tang FL, Wu DH, Lu ZG, Huang F, Zhou YX. The efficacy and safety of diacerein in treatmnet of painful osteoarthritis of the knee. 11º Asia Pacific League of Associations for Rheumatology Congress, Jeju Island, Korea. 2004:35.
Valat 1997 {published data only}
-
- Valat‐JP. Diacerein Pragmatic Study: Clinical, Life quality and socio‐ economic analysis [Étude pragmatique de la diacerheine: résultats sur les signes cliniques, la qualité de vie et les coûts médico‐économiques]. La Revue du Praticien 1997;47:S39‐S45. - PubMed
Vignon 2002 {published data only}
-
- Vignon E. Results of ECHODIAH study in hip osteoarthritis [Résultats de l'essai thérapeutique ECHODIAH dans l"arthrose de hanche]. La Presse Médicale 2002;31(1):7‐9. - PubMed
Villani 1998 {published data only}
-
- Villani P, Bouvenot G. Assessment of the placebo effect of symptomatic slow‐acting drugs given for osteoarthritis [Approche de l'intensité de l'effet placebo dans l'évaluation des anti‐arthrosiques symptomatiques d'action lente]. La Presse Médicale 1998;27(5):211‐214. - PubMed
Villermay 1994 {published data only}
-
- Villermay D. A new medicine for Arthrosis [Un Nouveau Médicament Pour L'Arthrose]. revue de L'Infirmière 1995;3:10‐11. - PubMed
References to studies awaiting assessment
Shin 2013 {published data only (unpublished sought but not used)}
-
- Kichul Shin, Joon Wan Kim, Ki won Moon, Ji Ae Yang, Eun Yeong Lee, MD, Yeong Wook Song, MD, Eun Bong Lee. The Efficacy of Diacerein in Hand Osteoarthritis:A Double‐Blind, Randomized, Placebo‐Controlled Study. Clinical Therapeutics 2013;35:431‐439. - PubMed
Additional references
ACR 2000
-
- American College of Rheumatology Siubcommittee on Osteoarthritis Guidelines. ACR recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.. Arthritis & Rheumatism 2000;43(9):1905‐15. - PubMed
Altman 1986
-
- Altman R, Asch E, Bloch D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee. Arthritis and Rheumatism 1986;29:1039‐49. - PubMed
Altman 1990
-
- Altman R, Alarcon G, Appelrough D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis and Rheumatism 1990;33:1601‐10. - PubMed
Bartels 2010
-
- Bartels EM, Bliddal H, Schondorff PK, Altman RD, Zhang W, Christensen R. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: meta‐analysis of randomized placebo‐controlled trials. Osteoarthritis and Cartilage 2010;18:289‐296. - PubMed
Bellamy 1988
-
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology 1988;15:1833‐40. - PubMed
Bellamy 1997
-
- Bellamy N, Kirwan J, Boers M. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip and hand osteoarthritis. Consensus development at OMERACT III. Journal of Rheumatology 1997;24:700‐802. - PubMed
Bellamy 2006
Berembaum 2010
-
- Berembaum F, Sellam J. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature Reviews Rheumatology 2010;11:625‐35. - PubMed
Bijlsma 2012
-
- Bijlsma JWJ. Textbook on Rheumatic Diseases. First Edition. London: BMJ Group, 2012.
Carlsson 1983
-
- Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983;16:87‐101. - PubMed
Dougados 2004
-
- Dougados M. Outcome Measures for Clinical Trials of Disease Modifying Osteoarthritis Drugs in Patients with Hip Osteoarthritis.. The Journal of Rheumatology 2004;70:66‐9. - PubMed
Dworkin 2011
-
- Dworkin RH, Pierce‐Sandner S, Turk DC, McDermott MP, Gibofsky A, Simon LS, Farrar JT, Katz NP. Outcome measures in placebo‐controlled trials of osteoarthritis: responsiveness to treatment effects in the REPORT database.. Osteoarthritis Cartilage 2011;19:483‐92. - PubMed
Egger 1997
-
- Egger M, Zellweger‐Zahner T, Scheider M, Junker C, Lengeler Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350:326‐9. - PubMed
Grade 2008
-
- Grading Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2008;336:924‐1051.
Hellio 2009
-
- Hellio Le Graverand‐Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed?. Osteoarthritis Cartilage 2009;17:1393‐401. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). www.cochrane‐handbook.org. UK: The Cochrane Collaboration, 2011.
Hochberg 2001
-
- Hochberg M C, Dougados M. Pharmacological therapy of osteoarthritis. Best Practice & Research Clinical Rheumatology 2001;15(4):583‐93. - PubMed
Hunter 2011
-
- Hunter DJ. Pharmacologic therapy for osteoarthritis ‐ the era of disease modification.. Nature Reviews Rheumatology 2011;7(1):13‐22. - PubMed
Kellgren 1957
Lawrence 1998
-
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis & Rheumatism 1998;41(4):778‐99. - PubMed
Lequesne 1987
-
- Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Validation ‐ value in comparison with other assessment tests.. Scandinavian Journal of Rheumatology 1987;(Suppl 65):85‐9. - PubMed
Lingetti 1982
-
- Lingetti M, D'Ambrosio PL, Grezia F, et al. A controlled study in the treatment of osteoarthritis with diacetylrhein (arthrodar). Current Therapeutic Research 1982;31(3):408‐12.
Murphy 2012
-
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population‐health perspective. American Journal of Nursing 2012;112(Suppl 1):S13‐9. - PubMed
Pelletier 2010
Pham 2003
-
- Pham T, Heijde DV, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome Variables for Osteoarthritis Clinical Trials: The OMERACT‐OARSI Set of Responder Criteria. Journal of Rheumatology 2003;30:1648‐54. - PubMed
Picavet 2003
PRAC 2013
-
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC). PRAC recommends suspension of diacerein‐containing medicines. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referr... 8 November 2013.
Rintelen 2006
-
- Rintelen B, Neumann K, Leeb BF. A Meta‐analysis of Controlled Clinical Studies With Diacerein in the Treatment of Osteoarthritis. Archives of Internal Medicine 2006;166:1899‐1902. - PubMed
Spencer 1997
-
- Spencer CM, Wilde MI. Diacerein. Drugs 1997;53(1):98‐106. - PubMed
Sun 2007
-
- Sun BH, Wu CW, Kalunian KC. New developments in osteoarthritis.. Rheumatic Diseases Clinic of North America 2007;33(1):135‐48. - PubMed
Towheed 2008
Towheed 2006
Woolf 2003
Zhang 2001
-
- Zhang Y, Xu L, Nevitt MC, Aliabadi P, Yu W, Qin M, et al. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: the Beijing Osteoarthritis Study. Arthritis & Rheumatism 2001;44(9):2065‐71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

