Evaluation of alternate categorical tumor metrics and cut points for response categorization using the RECIST 1.1 data warehouse
- PMID: 24516033
- PMCID: PMC3940541
- DOI: 10.1200/JCO.2013.52.3019
Evaluation of alternate categorical tumor metrics and cut points for response categorization using the RECIST 1.1 data warehouse
Abstract
Purpose: We sought to test and validate the predictive utility of trichotomous tumor response (TriTR; complete response [CR] or partial response [PR] v stable disease [SD] v progressive disease [PD]), disease control rate (DCR; CR/PR/SD v PD), and dichotomous tumor response (DiTR; CR/PR v others) metrics using alternate cut points for PR and PD. The data warehouse assembled to guide the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was used.
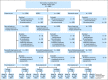
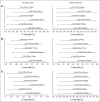
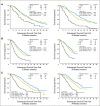
Methods: Data from 13 trials (5,480 patients with metastatic breast cancer, non-small-cell lung cancer, or colorectal cancer) were randomly split (60:40) into training and validation data sets. In all, 27 pairs of cut points for PR and PD were considered: PR (10% to 50% decrease by 5% increments) and PD (10% to 20% increase by 5% increments), for which 30% and 20% correspond to the RECIST categorization. Cox proportional hazards models with landmark analyses at 12 and 24 weeks stratified by study and number of lesions (fewer than three v three or more) and adjusted for average baseline tumor size were used to assess the impact of each metric on overall survival (OS). Model discrimination was assessed by using the concordance index (c-index).
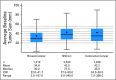
Results: Standard RECIST cut points demonstrated predictive ability similar to the alternate PR and PD cut points. Regardless of tumor type, the TriTR, DiTR, and DCR metrics had similar predictive performance. The 24-week metrics (albeit with higher c-index point estimate) were not meaningfully better than the 12-week metrics. None of the metrics did particularly well for breast cancer.
Conclusion: Alternative cut points to RECIST standards provided no meaningful improvement in OS prediction. Metrics assessed at 12 weeks have good predictive performance.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



References
-
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. - PubMed
-
- Bogaerts J, Ford R, Sargent D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–260. - PubMed
-
- Lavin PT. An alternative model for the evaluation of antitumor activity. Cancer Clin Trials. 1981;4:451–457. - PubMed
-
- Dhani N, Tu D, Sargent DJ, et al. Alternate endpoints for screening phase II studies. Clin Cancer Res. 2009;15:1873–1882. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

