Resveratrol and aspirin eliminate tetraploid cells for anticancer chemoprevention
- PMID: 24516128
- PMCID: PMC3939868
- DOI: 10.1073/pnas.1318440111
Resveratrol and aspirin eliminate tetraploid cells for anticancer chemoprevention
Abstract
Tetraploidy constitutes a genomically metastable state that can lead to aneuploidy and genomic instability. Tetraploid cells are frequently found in preneoplastic lesions, including intestinal cancers arising due to the inactivation of the tumor suppressor adenomatous polyposis coli (APC). Using a phenotypic screen, we identified resveratrol as an agent that selectively reduces the fitness of tetraploid cells by slowing down their cell cycle progression and by stimulating the intrinsic pathway of apoptosis. Selective killing of tetraploid cells was observed for a series of additional agents that indirectly or directly stimulate AMP-activated protein kinase (AMPK) including salicylate, whose chemopreventive action has been established by epidemiological studies and clinical trials. Both resveratrol and salicylate reduced the formation of tetraploid or higher-order polyploid cells resulting from the culture of human colon carcinoma cell lines or primary mouse epithelial cells lacking tumor protein p53 (TP53, best known as p53) in the presence of antimitotic agents, as determined by cytofluorometric and videomicroscopic assays. Moreover, oral treatment with either resveratrol or aspirin, the prodrug of salicylate, repressed the accumulation of tetraploid intestinal epithelial cells in the Apc(Min/+) mouse model of colon cancer. Collectively, our results suggest that the chemopreventive action of resveratrol and aspirin involves the elimination of tetraploid cancer cell precursors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
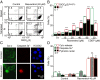
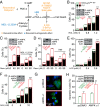
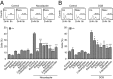

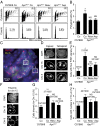
Comment in
-
Prevention: daily aspirin and chemoprevention.Nat Rev Clin Oncol. 2014 Apr;11(4):180. doi: 10.1038/nrclinonc.2014.35. Epub 2014 Feb 25. Nat Rev Clin Oncol. 2014. PMID: 24569449 No abstract available.
References
-
- Ganem NJ, Pellman D. Limiting the proliferation of polyploid cells. Cell. 2007;131(3):437–440. - PubMed
-
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet. 2012;13(3):189–203. - PubMed
-
- Storchova Z, Kuffer C. The consequences of tetraploidy and aneuploidy. J Cell Sci. 2008;121(Pt 23):3859–3866. - PubMed
-
- Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12(6):385–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

