G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding
- PMID: 24516170
- PMCID: PMC3939921
- DOI: 10.1073/pnas.1321436111
G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding
Abstract
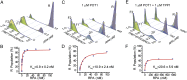
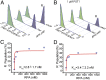
Human telomeres terminate with a single-stranded 3' G overhang, which can be recognized as a DNA damage site by replication protein A (RPA). The protection of telomeres (POT1)/POT1-interacting protein 1 (TPP1) heterodimer binds specifically to single-stranded telomeric DNA (ssTEL) and protects G overhangs against RPA binding. The G overhang spontaneously folds into various G-quadruplex (GQ) conformations. It remains unclear whether GQ formation affects the ability of POT1/TPP1 to compete against RPA to access ssTEL. Using single-molecule Förster resonance energy transfer, we showed that POT1 stably loads to a minimal DNA sequence adjacent to a folded GQ. At 150 mM K(+), POT1 loading unfolds the antiparallel GQ, as the parallel conformation remains folded. POT1/TPP1 loading blocks RPA's access to both folded and unfolded telomeres by two orders of magnitude. This protection is not observed at 150 mM Na(+), in which ssTEL forms only a less-stable antiparallel GQ. These results suggest that GQ formation of telomeric overhangs may contribute to suppression of DNA damage signals.
Keywords: DNA damage response; single molecule imaging; telomere protection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
POT1-TPP1 Binding and Unfolding of Telomere DNA Discriminates against Structural Polymorphism.J Mol Biol. 2016 Jul 3;428(13):2695-708. doi: 10.1016/j.jmb.2016.04.031. Epub 2016 May 10. J Mol Biol. 2016. PMID: 27173378 Free PMC article.
-
Coordinated interactions of multiple POT1-TPP1 proteins with telomere DNA.J Biol Chem. 2013 Jun 7;288(23):16361-16370. doi: 10.1074/jbc.M113.471896. Epub 2013 Apr 24. J Biol Chem. 2013. PMID: 23616058 Free PMC article.
-
TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase.Nature. 2007 Feb 1;445(7127):559-62. doi: 10.1038/nature05469. Epub 2007 Jan 21. Nature. 2007. PMID: 17237767
-
Structural biology of telomeres and telomerase.Cell Mol Life Sci. 2020 Jan;77(1):61-79. doi: 10.1007/s00018-019-03369-x. Epub 2019 Nov 14. Cell Mol Life Sci. 2020. PMID: 31728577 Free PMC article. Review.
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
Cited by
-
POT1 stability and binding measured by fluorescence thermal shift assays.PLoS One. 2021 Mar 30;16(3):e0245675. doi: 10.1371/journal.pone.0245675. eCollection 2021. PLoS One. 2021. PMID: 33784306 Free PMC article.
-
Modulation of DNA structure formation using small molecules.Biochim Biophys Acta Mol Cell Res. 2019 Dec;1866(12):118539. doi: 10.1016/j.bbamcr.2019.118539. Epub 2019 Sep 3. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31491448 Free PMC article. Review.
-
G4-quadruplex-binding proteins: review and insights into selectivity.Biophys Rev. 2022 Apr 20;14(3):635-654. doi: 10.1007/s12551-022-00952-8. eCollection 2022 Jun. Biophys Rev. 2022. PMID: 35791380 Free PMC article. Review.
-
Interrogating accessibility of telomeric sequences with FRET-PAINT: evidence for length-dependent telomere compaction.Nucleic Acids Res. 2021 Apr 6;49(6):3371-3380. doi: 10.1093/nar/gkab067. Nucleic Acids Res. 2021. PMID: 33693934 Free PMC article.
-
Dynamic interaction of BRCA2 with telomeric G-quadruplexes underlies telomere replication homeostasis.Nat Commun. 2022 Jun 13;13(1):3396. doi: 10.1038/s41467-022-31156-z. Nat Commun. 2022. PMID: 35697743 Free PMC article.
References
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. - PubMed
-
- Wold MS. Replication protein A: A heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. - PubMed
-
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300(5625):1542–1548. - PubMed
-
- Lei M, Baumann P, Cech TR. Cooperative binding of single-stranded telomeric DNA by the Pot1 protein of Schizosaccharomyces pombe. Biochemistry. 2002;41(49):14560–14568. - PubMed
-
- Loayza D, Parsons H, Donigian J, Hoke K, de Lange T. DNA binding features of human POT1: A nonamer 5′-TAGGGTTAG-3′ minimal binding site, sequence specificity, and internal binding to multimeric sites. J Biol Chem. 2004;279(13):13241–13248. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

