Shigella type III secretion protein MxiI is recognized by Naip2 to induce Nlrc4 inflammasome activation independently of Pkcδ
- PMID: 24516390
- PMCID: PMC3916413
- DOI: 10.1371/journal.ppat.1003926
Shigella type III secretion protein MxiI is recognized by Naip2 to induce Nlrc4 inflammasome activation independently of Pkcδ
Abstract
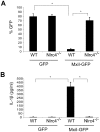
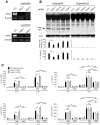
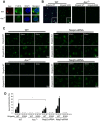
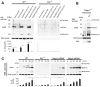
Recognition of intracellular pathogenic bacteria by members of the nucleotide-binding domain and leucine-rich repeat containing (NLR) family triggers immune responses against bacterial infection. A major response induced by several Gram-negative bacteria is the activation of caspase-1 via the Nlrc4 inflammasome. Upon activation, caspase-1 regulates the processing of proIL-1β and proIL-18 leading to the release of mature IL-1β and IL-18, and induction of pyroptosis. The activation of the Nlrc4 inflammasome requires the presence of an intact type III or IV secretion system that mediates the translocation of small amounts of flagellin or PrgJ-like rod proteins into the host cytosol to induce Nlrc4 activation. Using the Salmonella system, it was shown that Naip2 and Naip5 link flagellin and the rod protein PrgJ, respectively, to Nlrc4. Furthermore, phosphorylation of Nlrc4 at Ser533 by Pkcδ was found to be critical for the activation of the Nlrc4 inflammasome. Here, we show that Naip2 recognizes the Shigella T3SS inner rod protein MxiI and induces Nlrc4 inflammasome activation. The expression of MxiI in primary macrophages was sufficient to induce pyroptosis and IL-1β release, which were prevented in macrophages deficient in Nlrc4. In the presence of MxiI or Shigella infection, MxiI associated with Naip2, and Naip2 interacted with Nlrc4. siRNA-mediated knockdown of Naip2, but not Naip5, inhibited Shigella-induced caspase-1 activation, IL-1β maturation and Asc pyroptosome formation. Notably, the Pkcδ kinase was dispensable for caspase-1 activation and secretion of IL-1β induced by Shigella or Salmonella infection. These results indicate that activation of caspase-1 by Shigella is triggered by the rod protein MxiI that interacts with Naip2 to induce activation of the Nlrc4 inflammasome independently of the Pkcδ kinase.
Conflict of interest statement
Luigi Franchi is an employee of Lycera, a biotechnology company working in the area of inflammation and autoimmune disease. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures


References
-
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozören N, et al. (2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nat Immunol 7: 576–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

