Evaluation of stress tolerance and fermentative behavior of indigenous Saccharomyces cerevisiae
- PMID: 24516430
- PMCID: PMC3910215
- DOI: 10.1590/S1517-83822013005000051
Evaluation of stress tolerance and fermentative behavior of indigenous Saccharomyces cerevisiae
Abstract
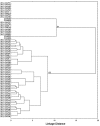
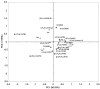
Sixty six indigenous Saccharomyces cerevisiae strains were evaluated in stressful conditions (temperature, osmolarity, sulphite and ethanol tolerance) and also ability to flocculate. Eighteen strains showed tolerant characteristics to these stressful conditions, growing at 42 °C, in 0.04% sulphite, 1 mol L(-1) NaCl and 12% ethanol. No flocculent characteristics were observed. These strains were evaluated according to their fermentative performance in sugar cane juice. The conversion factors of substrates into ethanol (Y(p/s)), glycerol (Y(g/s)) and acetic acid (Y(ac/s)), were calculated. The highest values of Y(p/s) in sugar cane juice fermentation were obtained by four strains, one isolated from fruit (0.46) and the others from sugar cane (0.45, 0.44 and 0.43). These values were higher than the value obtained using traditional yeast (0.38) currently employed in the Brazilian bioethanol industry. The parameters Y(g/s) and Y(ac/s) were low for all strains. The UFLA FW221 presented the higher values for parameter related to bioethanol production. Thus, it was tested in co-culture with Lactobacillus fermentum. Besides this, a 20-L vessel for five consecutive batches of fermentation was performed. This strain was genetically stable and remained viable during all batches, producing high amounts of ethanol. The UFLA FW221 isolated from fruit was suitable to produce bioethanol in sugar cane juice. Therefore, the study of the biodiversity of yeasts from different environmental can reveal strains with desired characteristics to industrial applications.
Keywords: Saccharomyces cerevisiae; UFLA FW221; alcoholic fermentation; biofuel; fermentation kinetics.
Figures





References
-
- Aguilera J, Randez-Gil F, Prieto JA. Cold response in Saccharomyces cerevisiae: new functions for old mechanisms. FEMS Microbiol Rev. 2007;31:327–341. - PubMed
-
- Andrietta SR, Steckelber C, Andrietta MGS. Study of flocculent yeast performance in tower reactors for bioethanol production in a continuous fermentation process with no cell recycling. Bioresource Technol. 2008;99:3002–3008. - PubMed
-
- Antoni D, Zeverlov VV, Schwarz WH. Biofuels from microbes. Appl Microbiol Biotechnol. 2007;77:23–35. - PubMed
-
- Attfield PV. Stress tolerance: the key to effective strains of industrial baker’s yeast. Nat Biotechnol. 1997;15:1351–1357. - PubMed
-
- Babiker MA, Abdel-Banat, Hoshida H, Ano A, Nonklang S, Akada R. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl Microbiol Biot. 2010;85:861–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
