Neural crest-derived mesenchymal cells require Wnt signaling for their development and drive invagination of the telencephalic midline
- PMID: 24516524
- PMCID: PMC3916303
- DOI: 10.1371/journal.pone.0086025
Neural crest-derived mesenchymal cells require Wnt signaling for their development and drive invagination of the telencephalic midline
Abstract
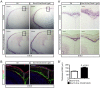
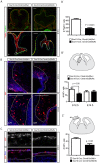
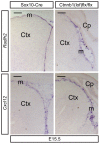
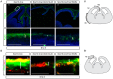
Embryonic neural crest cells contribute to the development of the craniofacial mesenchyme, forebrain meninges and perivascular cells. In this study, we investigated the function of ß-catenin signaling in neural crest cells abutting the dorsal forebrain during development. In the absence of ß-catenin signaling, neural crest cells failed to expand in the interhemispheric region and produced ectopic smooth muscle cells instead of generating dermal and calvarial mesenchyme. In contrast, constitutive expression of stabilized ß-catenin in neural crest cells increased the number of mesenchymal lineage precursors suggesting that ß-catenin signaling is necessary for the expansion of neural crest-derived mesenchymal cells. Interestingly, the loss of neural crest-derived mesenchymal stem cells (MSCs) leads to failure of telencephalic midline invagination and causes ventricular system defects. This study shows that ß-catenin signaling is required for the switch of neural crest cells to MSCs and mediates the expansion of MSCs to drive the formation of mesenchymal structures of the head. Furthermore, loss of these structures causes striking defects in forebrain morphogenesis.
Conflict of interest statement
Figures



References
-
- Trainor PA (2005) Specification of neural crest cell formation and migration in mouse embryos. Semin Cell Dev Biol 16: 683–693. - PubMed
-
- Currle DS, Cheng X, Hsu CM, Monuki ES (2005) Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development 132: 3549–3559. - PubMed
-
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW (1998) The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125: 2315–2325. - PubMed
-
- Miller JR, Moon RT (1996) Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev 10: 2527–2539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

