Global diversity and review of Siphonophorae (Cnidaria: Hydrozoa)
- PMID: 24516560
- PMCID: PMC3916360
- DOI: 10.1371/journal.pone.0087737
Global diversity and review of Siphonophorae (Cnidaria: Hydrozoa)
Erratum in
-
Correction: Global diversity and review of Siphonophorae (Cnidaria: Hydrozoa).PLoS One. 2015 Feb 6;10(2):e0118381. doi: 10.1371/journal.pone.0118381. eCollection 2015. PLoS One. 2015. PMID: 25658312 Free PMC article.
Abstract
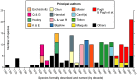
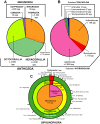
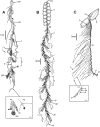
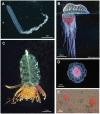
In this review the history of discovery of siphonophores, from the first formal description by Carl Linnaeus in 1785 to the present, is summarized, and species richness together with a summary of world-wide distribution of this pelagic group within the clade Hydrozoa discussed. Siphonophores exhibit three basic body plans which are briefly explained and figured, whilst other atypical body plans are also noted. Currently, 175 valid siphonophore species are recognized in the latest WoRMS world list, including 16 families and 65 genera. Much new information since the last review in 1987 is revealed from the first molecular analysis of the group, enabling identification of some new morphological characters diagnostic for physonect siphonophores. Ten types of nematocysts (stinging cells) are identified in siphonophores, more than in any other cnidarian; these are incorporated into batteries in the side branches of the tentacles in most species (here termed tentilla), and tentilla are reviewed in the last section of this paper. Their discharge mechanisms are explained and also how the tentilla of several physonect siphonophores are modified into lures. Of particular interest is the recent discovery of a previously unknown red fluorescent lure in the tentilla of the deep sea physonect Erenna, the first described example of emission of red light by an invertebrate to attract prey.
Conflict of interest statement
Figures
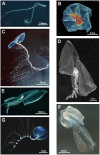
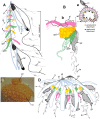
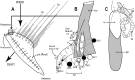



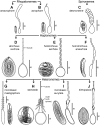




References
-
- WoRMS Siphonophora List. World Register of Marine Species website. Available: http://www.marinespecies.org/aphia.php?p=taxdetails&id=1371. Accessed 2014 Jan 6.
-
- Mackie GO, Pugh PR, Purcell JE (1987) Siphonophore Biology. Adv Mar Biol 24: 97–262.
-
- Robison BH (2004) Deep pelagic biology. J Exp Mar Biol Ecol 300: 253–272.
-
- Mapstone GM, Ljubenkov JC (2013) New observations on Dromalia alexandri Bigelow, 1911, a rhodaliid physonect siphonophore from southern Californian waters. Marine Ecology 34 (1): 96–112.
-
- Alvariño A (1971) Siphonophores of the Pacific with a review of the world distribution. Bull Scripps Inst Oceanogr Univ Calif Technical Series No. 16.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

