Cytocidal activities of topoisomerase 1 inhibitors and 5-azacytidine against pheochromocytoma/paraganglioma cells in primary human tumor cultures and mouse cell lines
- PMID: 24516563
- PMCID: PMC3917832
- DOI: 10.1371/journal.pone.0087807
Cytocidal activities of topoisomerase 1 inhibitors and 5-azacytidine against pheochromocytoma/paraganglioma cells in primary human tumor cultures and mouse cell lines
Erratum in
- PLoS One. 2014;9(3):e92492
Abstract
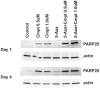
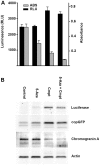
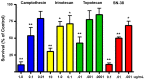
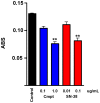
There is currently no effective treatment for metastatic pheochromocytomas and paragangliomas. A deficiency in current chemotherapy regimens is that the metastases usually grow very slowly. Drugs that target dividing tumor cells have therefore had limited success. To improve treatment, new strategies and valid experimental models are required for pre-clinical testing. However, development of models has itself been hampered by the absence of human pheochromocytoma/paraganglioma cell lines for cultures or xenografts. Topoisomerase 1 (TOP1) inhibitors are drugs that interfere with mechanisms that maintain DNA integrity during transcription in both quiescent and dividing cells. We used primary cultures of representative human tumors to establish the cytotoxicity of camptothecin, a prototypical TOP1 inhibitor, against non-dividing pheochromocytoma/paraganglioma cells, and then employed a mouse pheochromocytoma model (MPC) to show that efficacy of low concentrations of camptothecin and other TOP1 inhibitors is increased by intermittent coadministration of sub-toxic concentrations of 5-azacytidine, a DNA methylation inhibitor that modulates transcription. We then tested the same drugs against a clonal MPC derivative that expresses CMV reporter-driven luciferase and GFP, intended for in vivo drug testing. Unexpectedly, luciferase expression, bioluminescence and GFP expression were paradoxically increased by both camptothecin and SN38, the active metabolite of irinotecan, thereby masking cell death. Expression of chromogranin A, a marker for neuroendocrine secretory granules, was not increased, indicating that the drug effects on levels of luciferase and GFP are specific to the GFP-luciferase construct rather than generalized cellular responses. Our findings provide proof of principle for use of TOP1 inhibitors against pheochromocytoma/paraganglioma and suggest novel strategies for enhancing efficacy and reducing toxicity by optimizing the combination and timing of their use in conjunction with other drugs. The paradoxical effects of TOP1 inhibitors on luciferase and GFP dictate a need for caution in the use of CMV promoter-regulated constructs for cancer-related imaging studies.
Conflict of interest statement
Figures




References
-
- DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors (2004) Tumours of Endocrine Organs. Lyon: IARC Press.
-
- Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, et al. (2007) Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab 3: 92–102. - PubMed
-
- Strong VE, Kennedy T, Al-Ahmadie H, Tang L, Coleman J, et al. (2008) Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery 143: 759–768. - PubMed
-
- Tischler AS, Powers JF, Alroy J (2004) Animal models of pheochromocytoma. Histol Histopathol 19: 883–895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

