Influence of murine mesenchymal stem cells on proliferation, phenotype, vitality, and cytotoxicity of murine cytokine-induced killer cells in coculture
- PMID: 24516591
- PMCID: PMC3916358
- DOI: 10.1371/journal.pone.0088115
Influence of murine mesenchymal stem cells on proliferation, phenotype, vitality, and cytotoxicity of murine cytokine-induced killer cells in coculture
Abstract
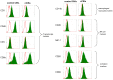
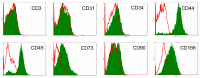
Stimulating lymphocytes with Ifn-γ, anti-CD3, and interleukin-2 promotes the proliferation of a cell population coexpressing T-lymphocyte surface antigens such as CD3, CD8a, and CD25 as well as natural killer cell markers such as NK1.1, CD49, and CD69. These cells, referred to as cytokine-induced killer cells (CIKs), display cytotoxic activity against tumour cells, even without prior antigen presentation, and offer a new cell-based approach to the treatment of malignant diseases. Because CIKs are limited in vivo, strategies to optimize in vitro culture yield are required. In the last 10 years, mesenchymal stem cells (MSCs) have gathered considerable attention. Aside from their uses in tissue engineering and as support in haematopoietic stem cell transplantations, MSCs show notable immunomodulatory characteristics, providing further possibilities for therapeutic applications. In this study, we investigated the influence of murine MSCs on proliferation, phenotype, vitality, and cytotoxicity of murine CIKs in a coculture system. We found that CIKs in coculture proliferated within 7 days, with an average growth factor of 18.84, whereas controls grew with an average factor of 3.7 in the same period. Furthermore, higher vitality was noted in cocultured CIKs than in controls. Cell phenotype was unaffected by coculture with MSCs and, notably, coculture did not impact cytotoxicity against the tumour cells analysed. The findings suggest that cell-cell contact is primarily responsible for these effects. Humoral interactions play only a minor role. Furthermore, no phenotypical MSCs were detected after coculture for 4 h, suggesting the occurrence of immune reactions between CIKs and MSCs. Further investigations with DiD-labelled MSCs revealed that the observed disappearance of MSCs appears not to be due to differentiation processes.
Conflict of interest statement
Figures






References
-
- Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS (2001) Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon y production. Blood 97: 2923–2931. - PubMed
-
- Märten A (2000) Tumorantigen gepulste dendritische Zellen zur Steigerung der Zytotoxizität immunologischer Effektorzellen bei Tumoren des gastroenteropankreatischen Systems [Dissertation]. Humboldt-Universität Berlin.
-
- Schmidt-Wolf IGH, Lefterova P, Mehta BA, Fernandez LP, Huhn D, et al. (1993) Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol 21: 1673–1679. - PubMed
-
- Introna M, Borleri G, Conti E, Franceschetti M, Barbui AM, et al. (2007) Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica 92: 952–959. - PubMed
-
- Laport GG, Sheehan K, Lowsky R, et al. (2006) Cytokine induced killer (CIK) cells as post-transplant immunotherapy following allogeneic hematopoietic cell transplantation. ASH Annual Meeting Abstracts 108: 412.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

