Relapse rates in patients with multiple sclerosis switching from interferon to fingolimod or glatiramer acetate: a US claims database study
- PMID: 24516663
- PMCID: PMC3916439
- DOI: 10.1371/journal.pone.0088472
Relapse rates in patients with multiple sclerosis switching from interferon to fingolimod or glatiramer acetate: a US claims database study
Abstract
Background: Approximately one-third of patients with multiple sclerosis (MS) are unresponsive to, or intolerant of, interferon (IFN) therapy, prompting a switch to other disease-modifying therapies. Clinical outcomes of switching therapy are unknown. This retrospective study assessed differences in relapse rates among patients with MS switching from IFN to fingolimod or glatiramer acetate (GA) in a real-world setting.
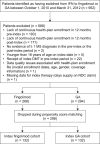
Methods: US administrative claims data from the PharMetrics Plus™ database were used to identify patients with MS who switched from IFN to fingolimod or GA between October 1, 2010 and March 31, 2012. Patients were matched 1∶1 using propensity scores within strata (number of pre-index relapses) on demographic (e.g. age and gender) and disease (e.g. timing of pre-index relapse, comorbidities and symptoms) characteristics. A claims-based algorithm was used to identify relapses while patients were persistent with therapy over 360 days post-switch. Differences in both the probability of experiencing a relapse and the annualized relapse rate (ARR) while persistent with therapy were assessed.
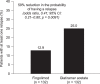
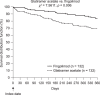
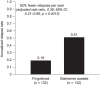
Results: The matched sample population contained 264 patients (n = 132 in each cohort). Before switching, 33.3% of patients in both cohorts had experienced at least one relapse. During the post-index persistence period, the proportion of patients with at least one relapse was lower in the fingolimod cohort (12.9%) than in the GA cohort (25.0%), and ARRs were lower with fingolimod (0.19) than with GA (0.51). Patients treated with fingolimod had a 59% lower probability of relapse (odds ratio, 0.41; 95% confidence interval [CI], 0.21-0.80; p = 0.0091) and 62% fewer relapses per year (rate ratio, 0.38; 95% CI, 0.21-0.68; p = 0.0013) compared with those treated with GA.
Conclusions: In a real-world setting, patients with MS who switched from IFNs to fingolimod were significantly less likely to experience relapses than those who switched to GA.
Conflict of interest statement
Figures
References
-
- Compston A, Coles A (2002) Multiple sclerosis. Lancet 359: 1221–1231. - PubMed
-
- Zwibel HL, Smrtka J (2011) Improving quality of life in multiple sclerosis: an unmet need. Am J Manag Care 17 Suppl 5 Improving: S139–145. - PubMed
-
- Bainbridge JL, Rieckmann P, editors (2008) Multiple sclerosis. In: DiPiro JT, Talbert R, Yee G, Matzke G, editors. Pharmacotherapy: a pathophysiologic approach. : New York: McGraw-Hill Medical. 913–926 p.
-
- Lublin FD, Baier M, Cutter G (2003) Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 61: 1528–1532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

