Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial
- PMID: 24517156
- PMCID: PMC4010689
- DOI: 10.1210/jc.2013-4440
Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial
Abstract
Context: Current osteoporosis medications increase bone mineral density (BMD) modestly and reduce, but do not eliminate, fracture risk. Attempts to improve efficacy by administering anabolic agents and bisphosphonates concomitantly have been unsuccessful. Conversely, 12 months of concomitant denosumab and teriparatide therapy increases BMD more than either drug alone.
Objective: The purpose of this study was to determine whether 24 months of combined denosumab and teriparatide will increase hip and spine BMD more than either individual agent.
Design: Preplanned continuation of the Denosumab and Teriparatide Administration (DATA) randomized controlled trial in which postmenopausal osteoporotic women received teriparatide (20 μg daily), denosumab (60 mg every 6 months), or both medications for 24 months.
Participants: Participants were 94 postmenopausal women with osteoporosis.
Outcome measures: Lumbar spine, femoral neck, total hip, and distal radius BMD and serum markers of bone turnover were measured.
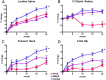
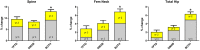
Results: At 24 months, lumbar spine BMD increased more in the combination group (12.9 ± 5.0%) than in either the teriparatide (9.5 ± 5.9%, P = .01) or denosumab (8.3 ± 3.4%, P = .008) groups. Femoral neck BMD also increased more in the combination group (6.8 ± 3.6%) than in either the teriparatide (2.8 ± 3.9%, P = .003) or denosumab (4.1 ± 3.8%, P = .008) groups. Similarly, total hip BMD increased more in the combination group (6.3 ± 2.6%) than in the teriparatide (2.0 ± 3.0%) or denosumab (3.2 ± 2.5%) groups (P < .001 for both). Although spine and hip BMD continued to increase in the second year in all groups, these year 2 increases did not differ among groups. Serum C-telopeptide and N-terminal propeptide of type 1 procollagen were equally suppressed in the denosumab and combination groups, whereas osteocalcin decreased more in the denosumab group than in the combination group, a difference that persisted, but lessened, in the second year of therapy.
Conclusions: Two years of concomitant teriparatide and denosumab therapy increases BMD more than therapy with either medication alone and more than has been reported with any current therapy. The combination of these agents may prove to be an important treatment option in patients at high risk of fracture.
Figures

Comment in
-
Letter to the Editor: Combination Treatment With Teriparatide and Denosumab in Osteoporosis.J Clin Endocrinol Metab. 2016 Aug;101(8):L80-1. doi: 10.1210/jc.2016-2343. J Clin Endocrinol Metab. 2016. PMID: 27486947 No abstract available.
References
-
- Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003;1:45–52 - PubMed
-
- Delmas PD. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom. 2008;11:325–338 - PubMed
-
- Dempster DW, Zhou H, Recker RR, et al. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:2799–2808 - PubMed
-
- Compston J. The use of combination therapy in the treatment of postmenopausal osteoporosis. Endocrine. 2012;41:11–18 - PubMed
-
- Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

