Tissue-based imaging model of human trabecular meshwork
- PMID: 24517246
- PMCID: PMC3991999
- DOI: 10.1089/jop.2013.0190
Tissue-based imaging model of human trabecular meshwork
Abstract
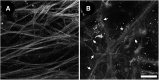
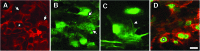
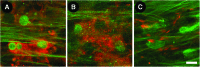
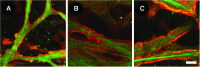
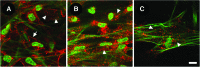
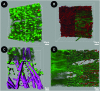
We have developed a tissue-based model of the human trabecular meshwork (TM) using viable postmortem corneoscleral donor tissue. Two-photon microscopy is used to optically section and image deep in the tissue to analyze cells and extracellular matrix (ECM) within the original three-dimensional (3D) environment of the TM. Multimodal techniques, including autofluorescence (AF), second harmonic generation (SHG), intravital dye fluorescence, and epifluorescence, are combined to provide unique views of the tissue at the cellular and subcellular level. SHG and AF imaging are non-invasive tissue imaging techniques with potential for clinical application, which can be modeled in the system. We describe the following in the tissue-based model: analysis of live cellularity to determine tissue viability; characteristics of live cells based on intravital labeling; features and composition of the TM's structural ECM; localization of specific ECM proteins to regions such as basement membrane; in situ induction and expression of tissue markers characteristic of cultured TM cells relevant to glaucoma; analysis of TM actin and pharmacological effects; in situ visualization of TM, inner wall endothelium, and Schlemm's canal; and application of 3D reconstruction, modeling, and quantitative analysis to the TM. The human model represents a cost-effective use of valuable and scarce yet available human tissue that allows unique cell biology, pharmacology, and translational studies of the TM.
Figures





Similar articles
-
Two-photon immunofluorescence characterization of the trabecular meshwork in situ.Invest Ophthalmol Vis Sci. 2012 Jun 5;53(7):3395-404. doi: 10.1167/iovs.11-8570. Invest Ophthalmol Vis Sci. 2012. PMID: 22531697 Free PMC article.
-
Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma.Exp Eye Res. 2015 Apr;133:112-25. doi: 10.1016/j.exer.2014.07.014. Exp Eye Res. 2015. PMID: 25819459 Free PMC article. Review.
-
Sources of structural autofluorescence in the human trabecular meshwork.Invest Ophthalmol Vis Sci. 2013 Jul 18;54(7):4813-20. doi: 10.1167/iovs.12-11235. Invest Ophthalmol Vis Sci. 2013. PMID: 23745000 Free PMC article.
-
Analyzing live cellularity in the human trabecular meshwork.Invest Ophthalmol Vis Sci. 2013 Feb 5;54(2):1039-47. doi: 10.1167/iovs.12-10479. Invest Ophthalmol Vis Sci. 2013. PMID: 23249706 Free PMC article.
-
The trabecular meshwork outflow pathways: structural and functional aspects.Exp Eye Res. 2009 Apr;88(4):648-55. doi: 10.1016/j.exer.2009.02.007. Epub 2009 Feb 23. Exp Eye Res. 2009. PMID: 19239914 Review.
Cited by
-
Ultrastructural variability of the juxtacanalicular tissue along the inner wall of Schlemm's canal.Mol Vis. 2019 Sep 21;25:517-526. eCollection 2019. Mol Vis. 2019. PMID: 31588175 Free PMC article.
-
Aqueous Humor Outflow Structure and Function Imaging At the Bench and Bedside: A Review.J Clin Exp Ophthalmol. 2016 Aug;7(4):578. doi: 10.4172/2155-9570.1000578. Epub 2016 Jul 24. J Clin Exp Ophthalmol. 2016. PMID: 27790380 Free PMC article.
-
Microstructure visualization of conventional outflow pathway and finite element modeling analysis of trabecular meshwork.Biomed Eng Online. 2016 Dec 28;15(Suppl 2):162. doi: 10.1186/s12938-016-0254-2. Biomed Eng Online. 2016. PMID: 28155681 Free PMC article.
-
Tissue-based multiphoton analysis of actomyosin and structural responses in human trabecular meshwork.Sci Rep. 2016 Feb 17;6:21315. doi: 10.1038/srep21315. Sci Rep. 2016. PMID: 26883567 Free PMC article.
-
Fibrillin-1 mutant mouse captures defining features of human primary open glaucoma including anomalous aqueous humor TGF beta-2.Sci Rep. 2022 Jun 23;12(1):10623. doi: 10.1038/s41598-022-14062-8. Sci Rep. 2022. PMID: 35739142 Free PMC article.
References
-
- Fuchshofer R., and Tamm E.R.Modulation of extracellular matrix turnover in the trabecular meshwork. Exp. Eye Res. 88:683–688, 2009 - PubMed
-
- Rhee D.J., Haddadin R.I., Kang M.H., and Oh D.J.Matricellular proteins in the trabecular meshwork. Exp. Eye Res. 88:694–703, 2009 - PubMed
-
- Polansky J.R., Wood I.S., Maglio M.T., and Alvarado J.A.Trabecular meshwork cell culture in glaucoma research: evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmology. 91:580–595, 1984 - PubMed
-
- Cukierman E., Pankov R., Stevens D.R., and Yamada K.M.Taking cell-matrix adhesions to the third dimension. Science. 294:1708–1712, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

