Sustained efficacy and safety of a 300IR daily dose of a sublingual solution of birch pollen allergen extract in adults with allergic rhinoconjunctivitis: results of a double-blind, placebo-controlled study
- PMID: 24517417
- PMCID: PMC3928083
- DOI: 10.1186/2045-7022-4-7
Sustained efficacy and safety of a 300IR daily dose of a sublingual solution of birch pollen allergen extract in adults with allergic rhinoconjunctivitis: results of a double-blind, placebo-controlled study
Abstract
Background: Allergic rhinoconjunctivitis (ARC) due to birch pollen is a growing health concern in Europe. Here, we report the efficacy and safety of 300IR birch pollen sublingual solution administered discontinuously for 2 consecutive years to patients with birch-associated allergic rhinoconjunctivitis.
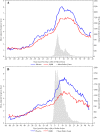
Methods: Birch pollen-allergic adults were randomized in this double blind study to 300IR birch pollen sublingual solution or placebo, daily, starting 4 months before and continuing through the pollen season for two pollen seasons. Randomization was stratified according to the presence or absence of oral allergy syndrome (OAS). The primary efficacy endpoint was the Average Adjusted Symptom Score (AAdSS) over the second pollen season and was analyzed by ANCOVA. Secondary efficacy endpoints included the AAdSS over the first pollen period. Safety was evaluated by means of adverse event monitoring.
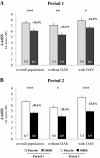
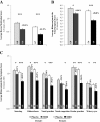
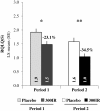
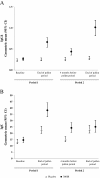
Results: 574 patients (284 in the active group and 290 in the placebo group) were randomized and 496 completed the study. Over the second pollen period, the least square (LS) mean AAdSS was significantly lower in the 300IR group than in the placebo group (LS mean difference -2.04, 95% CI [-2.69, -1.40], (p <0.0001) with a relative reduction of 30.6%. Results were consistent in patients with and without OAS (-33.6% and -28.4%, respectively). A significant reduction in LS mean AAdSS was also observed over the first pollen season. The most frequently reported adverse events were application site reactions: oral pruritus, throat irritation, and mouth edema. There were no reports of anaphylaxis.
Conclusions: Pre- and co-seasonal treatment with 300IR birch pollen sublingual solution demonstrated sustained clinical efficacy over 2 pollen seasons and was well tolerated in adults with birch pollen-associated allergic rhinoconjunctivitis. Efficacy results were consistent in patients with and without oral allergy syndrome.
Trial registration: ClinicalTrials.gov: NCT01731249.
Figures

References
-
- Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann NY Acad Sciences. 2002;964:47–68. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

