Desensitization for solid organ and hematopoietic stem cell transplantation
- PMID: 24517434
- PMCID: PMC4237559
- DOI: 10.1111/imr.12150
Desensitization for solid organ and hematopoietic stem cell transplantation
Abstract
Desensitization protocols are being used worldwide to enable kidney transplantation across immunologic barriers, i.e. antibody to donor HLA or ABO antigens, which were once thought to be absolute contraindications to transplantation. Desensitization protocols are also being applied to permit transplantation of HLA mismatched hematopoietic stem cells to patients with antibody to donor HLA, to enhance the opportunity for transplantation of non-renal organs, and to treat antibody-mediated rejection. Although desensitization for organ transplantation carries an increased risk of antibody-mediated rejection, ultimately these transplants extend and enhance the quality of life for solid organ recipients, and desensitization that permits transplantation of hematopoietic stem cells is life saving for patients with limited donor options. Complex patient factors and variability in treatment protocols have made it difficult to identify, precisely, the mechanisms underlying the downregulation of donor-specific antibodies. The mechanisms underlying desensitization may differ among the various protocols in use, although there are likely to be some common features. However, it is likely that desensitization achieves a sort of immune detente by first reducing the immunologic barrier and then by creating an environment in which an autoregulatory process restricts the immune response to the allograft.
Keywords: desensitization; donor-specific antibodies; hematopoietic stem cell transplantation; intravenous immunoglobulin; plasmapheresis; solid organ transplantation.
© 2014 The Authors. Immunological Reviews Published by John Wiley & Sons Ltd.
Figures

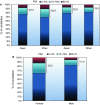
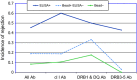
References
-
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2011 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. The data and analyses reported in the 2011 Annual Data Report of the Organ Procurement and Transplantation Network and the US Scientific Registry of Transplant Recipients have been supplied by the Minneapolis Medical Research Foundation and UNOS under contract with HHS/HRSA. The authors alone are responsible for reporting and interpreting these data; the views expressed herein are those of the authors and not necessarily those of the US Government.
-
- OPTN data as of September 20, 2013 for patients listed and transplanted between 2001-2002. 2013. Available from http://optn.transplant.hrsa.gov.
-
- Leffell MS, Steinberg AG, Bias WB, Machan CH, Zachary AA. The distribution of HLA antigens and phenotypes among donors and patients in the UNOS registry. Transplantation. 1994;58:1119–1130. - PubMed
-
- Zachary AA, Steinberg AG, Bias WB, Leffell MS. The frequencies of HLA alleles and haplotypes and their distribution among donors and renal patients in the UNOS registry. Transplantation. 1996;62:272–283. - PubMed
-
- Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994-2003; Department of Health and Human Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association, Ann Arbor, MI. 2004.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

