G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling
- PMID: 24518205
- PMCID: PMC4130805
- DOI: 10.1038/leu.2014.68
G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling
Abstract
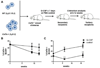
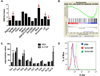
Recent studies demonstrate that inflammatory signals regulate hematopoietic stem cells (HSCs). Granulocyte colony-stimulating factor (G-CSF) is often induced with infection and has a key role in the stress granulopoiesis response. However, its effects on HSCs are less clear. Herein, we show that treatment with G-CSF induces expansion and increased quiescence of phenotypic HSCs, but causes a marked, cell-autonomous HSC repopulating defect associated with induction of Toll-like receptor (TLR) expression and signaling. The G-CSF-mediated expansion of HSCs is reduced in mice lacking TLR2, TLR4 or the TLR signaling adaptor MyD88. Induction of HSC quiescence is abrogated in mice lacking MyD88 or in mice treated with antibiotics to suppress intestinal flora. Finally, loss of TLR4 or germ-free conditions mitigates the G-CSF-mediated HSC repopulating defect. These data suggest that low-level TLR agonist production by commensal flora contributes to the regulation of HSC function and that G-CSF negatively regulates HSCs, in part, by enhancing TLR signaling.
Conflict of interest statement
Figures






References
-
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15(6):696–700. - PubMed
-
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

