Changes in the cholinergic innervation pattern of porcine ovaries with cysts induced by dexamethasone administration
- PMID: 24519145
- PMCID: PMC4125811
- DOI: 10.1007/s12031-014-0239-1
Changes in the cholinergic innervation pattern of porcine ovaries with cysts induced by dexamethasone administration
Abstract
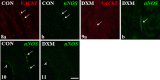
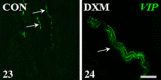
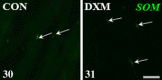
We revealed earlier that induction of ovarian cysts in gilts by dexamethasone phosphate disodium salt (DXM) administration from the follicular phase of the estrous cycle (EC) changed the cholinergic innervation of the gonad. In the present study, the innervation of porcine ovaries by vesicular acetylcholine transporter (VAChT)-, neuronal nitric oxide synthase (nNOS)-, vasoactive intestinal peptide (VIP)- and somatostatin (SOM)-immunoreactive (IR) fibres, after induction of cystic changes from the middle luteal phase of the EC, was determined. The cystic changes were induced by DXM injections from days 7 to 21 of the EC, and 11 days later, the ovaries were collected. In the cystic ovaries, VAChT-, nNOS- and SOM-IR fibres were found around cysts and small tertiary follicles; nNOS-IR and also VAChT-IR fibres were observed near secondary follicles and veins; and VAChT- and nNOS-IR fibres were not found around cortical arteries. The number of VIP-IR fibres increased near the cysts and within the ground plexus, while the number of VAChT-IR fibres decreased within the medullar part of this structure. Thus, our study showed changes in the cholinergic innervation pattern of the porcine cystic ovaries induced from the middle phase of the cycle and confirmed that cystic ovary innervation depends partly on the phase of the EC in which the induction of cysts was started.
Figures



Similar articles
-
Long-term estradiol-17β exposure decreases the cholinergic innervation pattern of the pig ovary.Ann Anat. 2018 Mar;216:135-141. doi: 10.1016/j.aanat.2017.11.010. Epub 2018 Jan 2. Ann Anat. 2018. PMID: 29305268
-
Long-term estradiol-17β administration changes the population of paracervical ganglion neurons supplying the ovary in adult gilts.J Mol Neurosci. 2013 Jul;50(3):424-33. doi: 10.1007/s12031-012-9950-y. Epub 2013 Jan 18. J Mol Neurosci. 2013. PMID: 23329259
-
Innervation pattern of polycystic ovaries in the women.J Chem Neuroanat. 2014 Nov;61-62:147-52. doi: 10.1016/j.jchemneu.2014.05.003. Epub 2014 Jun 4. J Chem Neuroanat. 2014. PMID: 24905277
-
Cholinergic innervation of the pancreas in the sheep.Acta Biol Hung. 2007 Jun;58(2):151-61. doi: 10.1556/ABiol.58.2007.2.2. Acta Biol Hung. 2007. PMID: 17585505
-
The noradrenergic innervation and steroidogenic activity of porcine cystic ovaries.Physiol Res. 2013;62(4):421-33. doi: 10.33549/physiolres.932471. Epub 2013 Apr 16. Physiol Res. 2013. PMID: 23590604
Cited by
-
Intrafollicular and Systemic Dopamine, Noradrenaline and Adrenaline Concentrations in Cycling Mares.Animals (Basel). 2020 Oct 16;10(10):1896. doi: 10.3390/ani10101896. Animals (Basel). 2020. PMID: 33081160 Free PMC article.
-
Muscarinic receptors in the rat ovary are involved in follicular development but not in steroid secretion.Physiol Rep. 2022 Nov;10(21):e15474. doi: 10.14814/phy2.15474. Physiol Rep. 2022. PMID: 36325585 Free PMC article.
References
-
- Ahmed Y, Akhtar AS, Qureshi F, Qureshi F, Anjum Q, Anhalt H. Polycystic ovarian syndrome: a new perspective. J Pak Med Assoc. 2003;53(2):72–77. - PubMed
-
- Andreani CL, Lazzarin N, Pierro E, Lanzone A, Mancuso S. Somatostatin action on rat ovarian steroidogenesis. Hum Reprod. 1995;10:1968–1973. - PubMed
-
- Barszczewska B, Jaroszewski JJ. The influence of nitric oxide on the contractile activity of the isolated porcine ovarian and uterine arteries. Pol J Vet Sci. 2004;7:83–90. - PubMed
-
- Bruno JB, Matos MHT, Chaves RN, Figueiredo JR. Involvement of vasoactive intestinal peptide (VIP) of ovarian physiology. Anim Reprod. 2011;8(3/4):51–57.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

