New insights into the role of the aryl hydrocarbon receptor in the function of CD11c⁺ cells during respiratory viral infection
- PMID: 24519489
- PMCID: PMC4048642
- DOI: 10.1002/eji.201343980
New insights into the role of the aryl hydrocarbon receptor in the function of CD11c⁺ cells during respiratory viral infection
Abstract
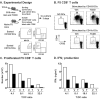
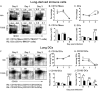
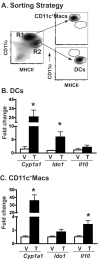
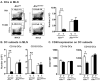
The aryl hydrocarbon receptor (AHR) has garnered considerable attention as a modulator of CD4(+) cell lineage development and function. It also regulates antiviral CD8(+) T-cell responses, but via indirect mechanisms that have yet to be determined. Here, we show that during acute influenza virus infection, AHR activation skews dendritic-cell (DC) subsets in the lung-draining lymph nodes, such that there are fewer conventional CD103(+) DCs and CD11b(+) DCs. Sorting DC subsets reveals AHR activation reduces immunostimulatory function of CD103(+) DCs in the mediastinal lymph nodes, and decreases their frequency in the lung. DNA-binding domain Ahr mutants demonstrate that alterations in DC subsets require the ligand-activated AHR to contain its inherent DNA-binding domain. To evaluate the intrinsic role of AHR in DCs, conditional knockouts were created using Cre-LoxP technology, which revealed that AHR in CD11c(+) cells plays a key role in controlling the acquisition of effector CD8(+) T cells in the infected lung. However, AHR within other leukocyte lineages contributes to diminished naïve CD8(+) T-cell activation in the draining lymphoid nodes. These findings indicate DCs are among the direct targets of AHR ligands in vivo, and AHR signaling modifies host responses to a common respiratory pathogen by affecting the complex interplay of multiple cell types.
Keywords: Antiviral immunity; CD8+ T cells; Dendritic cells (DCs); Influenza A virus.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



Similar articles
-
Environmental cues received during development shape dendritic cell responses later in life.PLoS One. 2018 Nov 9;13(11):e0207007. doi: 10.1371/journal.pone.0207007. eCollection 2018. PLoS One. 2018. PMID: 30412605 Free PMC article.
-
Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells.J Exp Med. 2008 Jul 7;205(7):1621-34. doi: 10.1084/jem.20071365. J Exp Med. 2008. PMID: 18591406 Free PMC article.
-
Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection.Toxicol Sci. 2010 Aug;116(2):514-22. doi: 10.1093/toxsci/kfq153. Epub 2010 May 23. Toxicol Sci. 2010. PMID: 20498003 Free PMC article.
-
Subsets of migrating intestinal dendritic cells.Immunol Rev. 2010 Mar;234(1):259-67. doi: 10.1111/j.0105-2896.2009.00866.x. Immunol Rev. 2010. PMID: 20193024 Review.
-
Therapeutic induction of tolerogenic dendritic cells via aryl hydrocarbon receptor signaling.Curr Opin Immunol. 2021 Jun;70:33-39. doi: 10.1016/j.coi.2021.02.003. Epub 2021 Feb 16. Curr Opin Immunol. 2021. PMID: 33607496 Review.
Cited by
-
Linking the aryl hydrocarbon receptor with altered DNA methylation patterns and developmentally induced aberrant antiviral CD8+ T cell responses.J Immunol. 2015 May 1;194(9):4446-57. doi: 10.4049/jimmunol.1402044. Epub 2015 Mar 25. J Immunol. 2015. PMID: 25810390 Free PMC article.
-
Environmental cues received during development shape dendritic cell responses later in life.PLoS One. 2018 Nov 9;13(11):e0207007. doi: 10.1371/journal.pone.0207007. eCollection 2018. PLoS One. 2018. PMID: 30412605 Free PMC article.
-
Role of aryl hydrocarbon receptors in infection and inflammation.Front Immunol. 2024 Apr 12;15:1367734. doi: 10.3389/fimmu.2024.1367734. eCollection 2024. Front Immunol. 2024. PMID: 38680494 Free PMC article. Review.
-
Reshaping the tumor microenvironment with oncolytic viruses, positive regulation of the immune synapse, and blockade of the immunosuppressive oncometabolic circuitry.J Immunother Cancer. 2022 Jul;10(7):e004935. doi: 10.1136/jitc-2022-004935. J Immunother Cancer. 2022. PMID: 35902132 Free PMC article.
-
Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity.Sci Rep. 2016 Apr 12;6:23820. doi: 10.1038/srep23820. Sci Rep. 2016. PMID: 27068235 Free PMC article.
References
-
- Denison M, Nagy S. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Phramacol. Toxicol. 2003;43:309–334. - PubMed
-
- Lawrence BP, Kerkvliet NI. Immune modulation by TCDD and related polyhalogenated aromatic hydrocarbones. In: Luebke R, House R, Kimber I, editors. Immunotoxicology and Immumopharmacology. 3 Edn CRC Press, Taylor & Francis Group; Boca Raton, FL: 2006. pp. 239–258.
-
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

