A reversible light-operated nanovalve on mesoporous silica nanoparticles
- PMID: 24519642
- PMCID: PMC4305341
- DOI: 10.1039/c3nr06049g
A reversible light-operated nanovalve on mesoporous silica nanoparticles
Abstract
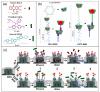
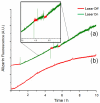
Two azobenzene α-cyclodextrin based nanovalves are designed, synthesized and assembled on mesoporous silica nanoparticles. Under aqueous conditions, the cyclodextrin cap is tightly bound to the azobenzene moiety and capable of holding back loaded cargo molecules. Upon irradiation with a near-UV light laser, trans to cis-photoisomerization of azobenzene initiates a dethreading process, which causes the cyclodextrin cap to unbind followed by the release of cargo. The addition of a bulky stopper to the end of the stalk allows this design to be reversible; complete dethreading of cyclodextrin as a result of unbinding with azobenzene is prevented as a consequence of steric interference. As a result, thermal relaxation of cis- to trans-azobenzene allows for the rebinding of cyclodextrin and resealing of the nanopores, a process which entraps the remaining cargo. Two stalks were designed with different lengths and tested with alizarin red S and propidium iodide. No cargo release was observed prior to light irradiation, and the system was capable of multiuse. On/off control was also demonstrated by monitoring the release of cargo when the light stimulus was applied and removed, respectively.
Figures


References
-
- Lu Y, Ganguli R, Drewien CA, Anderson MT, Brinker CJ, Gong W, Guo Y, Soyez H, Dunn B, Huang MH, Zink JI. Nature. 1997;389:364–368.
- Slowing II, Trewyn GB, Giri S, Lin SV. Adv. Funct. Mater. 2007;17:1225–1236.
- Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF. Science. 1998;279:548–552. - PubMed
- Lin Y-S, Hurley KR, Haynes CL. J. Phys. Chem. Lett. 2012;3:364–374. - PubMed
- Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Chem. Soc. Rev. 2012;41:2590–2605. - PubMed
-
- Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Nature. 1992;359:710–712.
-
- Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW. J. Am. Chem. Soc. 1992;114:10834–10843.
-
- Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889–896. - PMC - PubMed
- Ambrogio MW, Thomas CR, Zhao Y-L, Zink JI, Stoddart JF. Acc. Chem. Res. 2011;44:903–913. - PMC - PubMed
- Trewyn BG, Slowing II, Giri S, Chen H-T, Lin VSY. Acc. Chem. Res. 2007;40:846–853. - PubMed
- Zhang H, Dunphy DR, Jiang X, Meng H, Sun B, Tarn D, Xue M, Wang X, Lin S, Ji Z, Li R, Garcia FL, Yang J, Kirk M, Xia T, Zink JI, Nel AE, Brinker CJ. J. Am. Chem. Soc. 2012;134:15790–15804. - PMC - PubMed
- Yanes RE, Tarn D, Hwang AA, Ferris DP, Sherman SP, Thomas CR, Lu J, Pyle AD, Zink JI, Tamanoi F. Small. 2013;9:697–704. - PMC - PubMed
-
- Lu J, Liong M, Zink JI, Tamanoi F. Small. 2007;3:1341–1346. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

