Ultrasonography for clinical decision-making and intervention in airway management: from the mouth to the lungs and pleurae
- PMID: 24519789
- PMCID: PMC3999368
- DOI: 10.1007/s13244-014-0309-5
Ultrasonography for clinical decision-making and intervention in airway management: from the mouth to the lungs and pleurae
Abstract
Objectives: To create a state-of-the-art overview of the new and expanding role of ultrasonography in clinical decision-making, intervention and management of the upper and lower airways, that is clinically relevant, up-to-date and practically useful for clinicians.
Methods: This is a narrative review combined with a structured Medline literature search.
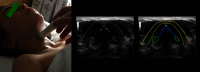
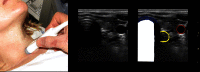
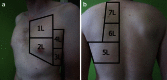
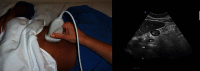
Results: Ultrasonography can be utilised to predict airway difficulty during induction of anaesthesia, evaluate if the stomach is empty or possesses gastric content that poses an aspiration risk, localise the essential cricothyroid membrane prior to difficult airway management, perform nerve blocks for awake intubation, confirm tracheal or oesophageal intubation and facilitate localisation of tracheal rings for tracheostomy. Ultrasonography is an excellent diagnostic tool in intraoperative and emergency diagnosis of pneumothorax. It also enables diagnosis and treatment of interstitial syndrome, lung consolidation, atelectasis, pleural effusion and differentiates causes of acute breathlessness during pregnancy. Patient safety can be enhanced by performing procedures under ultrasound guidance, e.g. thoracocentesis, vascular line access and help guide timing of removal of chest tubes by quantification of residual pneumothorax size.
Conclusions: Ultrasonography used in conjunction with hands-on management of the upper and lower airways has multiple advantages. There is a rapidly growing body of evidence showing its benefits.
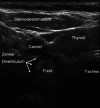
Teaching points: • Ultrasonography is becoming essential in management of the upper and lower airways. • The tracheal structures can be identified by ultrasonography, even when unidentifiable by palpation. • Ultrasonography is the primary diagnostic approach in suspicion of intraoperative pneumothorax. • Point-of-care ultrasonography of the airways has a steep learning curve. • Lung ultrasonography allows treatment of interstitial syndrome, consolidation, atelectasis and effusion.
Figures











References
-
- Cook TM, Woodall N, Harper J, Benger J. Major complications of airway management in the UK: results of the fourth national audit project of the royal college of anaesthetists and the difficult airway society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106:632–642. - PubMed
-
- Lichtenstein D, Meziere G, Biderman P, Gepner A, Barre O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. - PubMed
-
- Baldi G, Gargani L, Abramo A, et al. Lung water assessment by lung ultrasonography in intensive care: a pilot study. Intensive Care Med. 2013;39:74–84. - PubMed
-
- Soldati G, Inchingolo R, Smargiassi A, et al. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol. 2012;38:1169–1179. - PubMed
-
- Via G, Lichtenstein D, Mojoli F, et al. Whole lung lavage: a unique model for ultrasound assessment of lung aeration changes. Intensive Care Med. 2010;36:999–1007. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

