Glucose transporter 8 (GLUT8) mediates fructose-induced de novo lipogenesis and macrosteatosis
- PMID: 24519932
- PMCID: PMC4036240
- DOI: 10.1074/jbc.M113.527002
Glucose transporter 8 (GLUT8) mediates fructose-induced de novo lipogenesis and macrosteatosis
Abstract
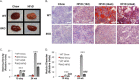
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in the world, and it is thought to be the hepatic manifestation of the metabolic syndrome. Excess dietary fructose causes both metabolic syndrome and NAFLD in rodents and humans, but the pathogenic mechanisms of fructose-induced metabolic syndrome and NAFLD are poorly understood. GLUT8 (Slc2A8) is a facilitative glucose and fructose transporter that is highly expressed in liver, heart, and other oxidative tissues. We previously demonstrated that female mice lacking GLUT8 exhibit impaired first-pass hepatic fructose metabolism, suggesting that fructose transport into the hepatocyte, the primary site of fructose metabolism, is in part mediated by GLUT8. Here, we tested the hypothesis that GLUT8 is required for hepatocyte fructose uptake and for the development of fructose-induced NAFLD. We demonstrate that GLUT8 is a cell surface-localized transporter and that GLUT8 overexpression or GLUT8 shRNA-mediated gene silencing significantly induces and blocks radiolabeled fructose uptake in cultured hepatocytes. We further show diminished fructose uptake and de novo lipogenesis in fructose-challenged GLUT8-deficient hepatocytes. Finally, livers from long term high-fructose diet-fed GLUT8-deficient mice exhibited attenuated fructose-induced hepatic triglyceride and cholesterol accumulation without changes in hepatocyte insulin-stimulated Akt phosphorylation. GLUT8 is thus essential for hepatocyte fructose transport and fructose-induced macrosteatosis. Fructose delivery across the hepatocyte membrane is thus a proximal, modifiable disease mechanism that may be exploited to prevent NAFLD.
Keywords: Diabetes; Fructose Metabolism; Glucose Transport; Hepatocyte; Metabolic Syndrome.
Figures





References
-
- Lim J. S., Mietus-Snyder M., Valente A., Schwarz J. M., Lustig R. H. (2010) The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 7, 251–264 - PubMed
-
- Pascale A., Pais R., Ratziu V. (2010) An Overview of Nonalcoholic Steatohepatitis: Past Present and Future Directions. J. Gastrointestin. Liver Dis. 19, 415–423 - PubMed
-
- Anstee Q. M., Targher G., Day C. P. (2013) Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HD040390-07/HD/NICHD NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- K12HD000850-29/HD/NICHD NIH HHS/United States
- K12 HD076224/HD/NICHD NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- T32 HD049305/HD/NICHD NIH HHS/United States
- R01 DK078187/DK/NIDDK NIH HHS/United States
- R01 HD040390/HD/NICHD NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- P30 DK52574/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

