Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis
- PMID: 24519940
- PMCID: PMC3975009
- DOI: 10.1074/jbc.M113.526517
Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis
Abstract
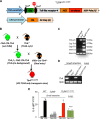
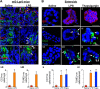
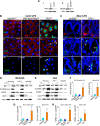
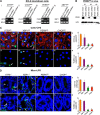
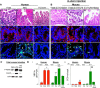
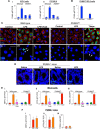
The cellular cues that regulate the apoptosis of intestinal stem cells (ISCs) remain incompletely understood, yet may play a role in diseases characterized by ISC loss including necrotizing enterocolitis (NEC). Toll-like receptor-4 (TLR4) was recently found to be expressed on ISCs, where its activation leads to ISC apoptosis through mechanisms that remain incompletely explained. We now hypothesize that TLR4 induces endoplasmic reticulum (ER) stress within ISCs, leading to their apoptosis in NEC pathogenesis, and that high ER stress within the premature intestine predisposes to NEC development. Using transgenic mice and cultured enteroids, we now demonstrate that TLR4 induces ER stress within Lgr5 (leucine-rich repeat-containing G-protein-coupled receptor 5)-positive ISCs, resulting in crypt apoptosis. TLR4 signaling within crypts was required, because crypt ER stress and apoptosis occurred in TLR4(ΔIEC-OVER) mice expressing TLR4 only within intestinal crypts and epithelium, but not TLR4(ΔIEC) mice lacking intestinal TLR4. TLR4-mediated ER stress and apoptosis of ISCs required PERK (protein kinase-related PKR-like ER kinase), CHOP (C/EBP homologous protein), and MyD88 (myeloid differentiation primary response gene 88), but not ATF6 (activating transcription factor 6) or XBP1 (X-box-binding protein 1). Human and mouse NEC showed high crypt ER stress and apoptosis, whereas genetic inhibition of PERK or CHOP attenuated ER stress, crypt apoptosis, and NEC severity. Strikingly, using intragastric delivery into fetal mouse intestine, prevention of ER stress reduced TLR4-mediated ISC apoptosis and mucosal disruption. These findings identify a novel link between TLR4-induced ER stress and ISC apoptosis in NEC pathogenesis and suggest that increased ER stress within the premature bowel predisposes to NEC development.
Keywords: Inflammation; Innate Immunity; Necrotizing Enterocolitis; Sepsis; Toll-like Receptors (TLR).
Figures



References
-
- Simons B. D., Clevers H. (2011) Stem cell self-renewal in intestinal crypt. Exp. Cell Res. 317, 2719–2724 - PubMed
-
- Sodhi C. P., Shi X. H., Richardson W. M., Grant Z. S., Shapiro R. A., Prindle T., Jr., Branca M., Russo A., Gribar S. C., Ma C., Hackam D. J. (2010) Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 - PMC - PubMed
-
- Feng J., Besner G. E. (2007) Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J. Pediatr. Surg. 42, 214–220 - PubMed
-
- Leaphart C. L., Cavallo J., Gribar S. C., Cetin S., Li J., Branca M. F., Dubowski T. D., Sodhi C. P., Hackam D. J. (2007) A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK083541/DK/NIDDK NIH HHS/United States
- DK0034854/DK/NIDDK NIH HHS/United States
- K08 DK101753/DK/NIDDK NIH HHS/United States
- R01 DK083752/DK/NIDDK NIH HHS/United States
- P50 GM053789/GM/NIGMS NIH HHS/United States
- 1R01GM078238/GM/NIGMS NIH HHS/United States
- R01 DK083541/DK/NIDDK NIH HHS/United States
- R01DK088199/DK/NIDDK NIH HHS/United States
- R01 GM078238/GM/NIGMS NIH HHS/United States
- P50GM053789/GM/NIGMS NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- R01 DK088199/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- 1R01DK083752/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

