Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo
- PMID: 24519956
- PMCID: PMC3996673
- DOI: 10.18632/oncotarget.1770
Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo
Abstract
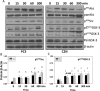
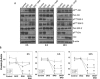
Recent studies suggest a positive correlation between glycogen synthase kinase-3 (GSK-3) activation and tumor growth. Currently, it is unclear how both Akt that inhibits GSK-3 and active GSK-3 are maintained concurrently in tumor cells. We investigated the role of GSK-3 and the existence of an Akt-resistant pathway for GSK-3 activation in prostate cancer cells. Our data show that Src, a non-receptor tyrosine kinase is responsible for Y216GSK-3 phosphorylation leading to its activation even when Akt is active. Experiments involving mouse embryonic fibroblasts lacking cSrc, Yes and Fyn, as well as Src activity modulation in prostate cancer cells with constitutively active (CA-Src) and dominant negative Src (DN-Src) plasmids demonstrated the integral role of Src in Y216GSK-3 phosphorylation and activity modulation. Inhibition of GSK-3 with SB415286 in PC3 cells resulted in impaired motility, proliferation and colony formation. Treatment of PC3 cells with the Src inhibitor dasatinib reduced Y216GSK-3 phosphorylation and inhibited proliferation, invasion and micrometastasis in vitro. Dasatinib treatment of athymic nude mice resulted in impaired growth of PC3 cell tumor xenograft. Together, we provide novel insight into the Src-mediated Y216GSK-3 phosphorylation and activation in prostate cancer cells and reveal the potential benefits of targeting Src-GSK-3 axis using drugs such as dasatinib.
Figures





Similar articles
-
Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases.Oncogene. 2010 Jun 3;29(22):3208-16. doi: 10.1038/onc.2010.103. Epub 2010 Apr 12. Oncogene. 2010. PMID: 20383201 Free PMC article.
-
Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model.Cancer Res. 2008 May 1;68(9):3323-33. doi: 10.1158/0008-5472.CAN-07-2997. Cancer Res. 2008. PMID: 18451159
-
The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer.J Natl Cancer Inst. 2011 Mar 2;103(5):407-24. doi: 10.1093/jnci/djq569. Epub 2011 Jan 31. J Natl Cancer Inst. 2011. PMID: 21282564
-
The role of Src in prostate cancer.Ann Oncol. 2007 Nov;18(11):1765-73. doi: 10.1093/annonc/mdm086. Epub 2007 Apr 10. Ann Oncol. 2007. PMID: 17426060 Review.
-
Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors.Clin Cancer Res. 2011 Sep 1;17(17):5546-52. doi: 10.1158/1078-0432.CCR-10-2616. Epub 2011 Jun 13. Clin Cancer Res. 2011. PMID: 21670084 Review.
Cited by
-
Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro.Sci Rep. 2016 May 19;6:26277. doi: 10.1038/srep26277. Sci Rep. 2016. PMID: 27195613 Free PMC article.
-
Decrypting a path based approach for identifying the interplay between PI3K and GSK3 signaling cascade from the perspective of cancer.Genes Dis. 2022 Feb 22;9(4):868-888. doi: 10.1016/j.gendis.2021.12.025. eCollection 2022 Jul. Genes Dis. 2022. PMID: 35685456 Free PMC article. Review.
-
Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer.Cell Death Dis. 2016 Feb 25;7(2):e2111. doi: 10.1038/cddis.2015.403. Cell Death Dis. 2016. PMID: 26913603 Free PMC article.
-
TOPK promotes metastasis of esophageal squamous cell carcinoma by activating the Src/GSK3β/STAT3 signaling pathway via γ-catenin.BMC Cancer. 2019 Dec 30;19(1):1264. doi: 10.1186/s12885-019-6453-z. BMC Cancer. 2019. PMID: 31888532 Free PMC article.
-
Suppression of Akt1-β-catenin pathway in advanced prostate cancer promotes TGFβ1-mediated epithelial to mesenchymal transition and metastasis.Cancer Lett. 2017 Aug 28;402:177-189. doi: 10.1016/j.canlet.2017.05.028. Epub 2017 Jun 7. Cancer Lett. 2017. PMID: 28602980 Free PMC article.
References
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. - PubMed
-
- Goc A, Al-Husein B, Kochuparambil ST, Liu J, Heston WW, Somanath PR. PI3 kinase integrates Akt and MAP kinase signaling pathways in the regulation of prostate cancer. Int J Oncol. 2011;38(1):267–277. - PubMed
-
- Kochuparambil ST, Al-Husein B, Goc A, Soliman S, Somanath PR. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J Pharmacol Exp Ther. 2011;336(2):496–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

