Nerve growth factor metabolic dysfunction in Down's syndrome brains
- PMID: 24519975
- PMCID: PMC3927704
- DOI: 10.1093/brain/awt372
Nerve growth factor metabolic dysfunction in Down's syndrome brains
Abstract
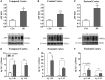
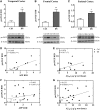
Basal forebrain cholinergic neurons play a key role in cognition. This neuronal system is highly dependent on NGF for its synaptic integrity and the phenotypic maintenance of its cell bodies. Basal forebrain cholinergic neurons progressively degenerate in Alzheimer's disease and Down's syndrome, and their atrophy contributes to the manifestation of dementia. Paradoxically, in Alzheimer's disease brains, the synthesis of NGF is not affected and there is abundance of the NGF precursor, proNGF. We have shown that this phenomenon is the result of a deficit in NGF's extracellular metabolism that compromises proNGF maturation and exacerbates its subsequent degradation. We hypothesized that a similar imbalance should be present in Down's syndrome. Using a combination of quantitative reverse transcription-polymerase chain reaction, enzyme-linked immunosorbent assay, western blotting and zymography, we investigated signs of NGF metabolic dysfunction in post-mortem brains from the temporal (n = 14), frontal (n = 34) and parietal (n = 20) cortex obtained from subjects with Down's syndrome and age-matched controls (age range 31-68 years). We further examined primary cultures of human foetal Down's syndrome cortex (17-21 gestational age weeks) and brains from Ts65Dn mice (12-22 months), a widely used animal model of Down's syndrome. We report a significant increase in proNGF levels in human and mouse Down's syndrome brains, with a concomitant reduction in the levels of plasminogen and tissue plasminogen activator messenger RNA as well as an increment in neuroserpin expression; enzymes that partake in proNGF maturation. Human Down's syndrome brains also exhibited elevated zymogenic activity of MMP9, the major NGF-degrading protease. Our results indicate a failure in NGF precursor maturation in Down's syndrome brains and a likely enhanced proteolytic degradation of NGF, changes which can compromise the trophic support of basal forebrain cholinergic neurons. The alterations in proNGF and MMP9 were also present in cultures of Down's syndrome foetal cortex; suggesting that this trophic compromise may be amenable to rescue, before frank dementia onset. Our study thus provides a novel paradigm for cholinergic neuroprotection in Alzheimer's disease and Down's syndrome.
Keywords: Alzheimer’s disease; Down’s syndrome; basal forebrain cholinergic neurons; matrix metallo-protease 9; proNGF.
Figures





Similar articles
-
Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion.Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10439-44. doi: 10.1073/pnas.181219298. Epub 2001 Aug 14. Proc Natl Acad Sci U S A. 2001. PMID: 11504920 Free PMC article.
-
Regional alterations in amyloid precursor protein and nerve growth factor across age in a mouse model of Down's syndrome.Neurosci Res. 2003 Apr;45(4):437-45. doi: 10.1016/s0168-0102(03)00005-1. Neurosci Res. 2003. PMID: 12657457
-
Nerve growth factor metabolic dysfunction in Alzheimer's disease and Down syndrome.Trends Pharmacol Sci. 2014 Jul;35(7):338-48. doi: 10.1016/j.tips.2014.04.010. Epub 2014 Jun 21. Trends Pharmacol Sci. 2014. PMID: 24962069 Review.
-
Nerve growth factor (NGF) pathway biomarkers in Down syndrome prior to and after the onset of clinical Alzheimer's disease: A paired CSF and plasma study.Alzheimers Dement. 2021 Apr;17(4):605-617. doi: 10.1002/alz.12229. Epub 2020 Nov 23. Alzheimers Dement. 2021. PMID: 33226181 Free PMC article.
-
A dual mechanism linking NGF/proNGF imbalance and early inflammation to Alzheimer's disease neurodegeneration in the AD11 anti-NGF mouse model.CNS Neurol Disord Drug Targets. 2011 Aug;10(5):635-47. doi: 10.2174/187152711796235032. CNS Neurol Disord Drug Targets. 2011. PMID: 21631402 Review.
Cited by
-
The Brain NGF Metabolic Pathway in Health and in Alzheimer's Pathology.Front Neurosci. 2019 Feb 12;13:62. doi: 10.3389/fnins.2019.00062. eCollection 2019. Front Neurosci. 2019. PMID: 30809111 Free PMC article. Review.
-
Down syndrome and Alzheimer's disease: Common pathways, common goals.Alzheimers Dement. 2015 Jun;11(6):700-9. doi: 10.1016/j.jalz.2014.10.007. Epub 2014 Dec 12. Alzheimers Dement. 2015. PMID: 25510383 Free PMC article.
-
Ghrelin ameliorates nerve growth factor Dysmetabolism and inflammation in STZ-induced diabetic rats.Metab Brain Dis. 2017 Jun;32(3):903-912. doi: 10.1007/s11011-017-0001-9. Epub 2017 Mar 30. Metab Brain Dis. 2017. PMID: 28357639
-
Cognitive Impairment, Neuroimaging, and Alzheimer Neuropathology in Mouse Models of Down Syndrome.Curr Alzheimer Res. 2016;13(1):35-52. doi: 10.2174/1567205012666150921095505. Curr Alzheimer Res. 2016. PMID: 26391050 Free PMC article. Review.
-
Hippocampal plasticity during the progression of Alzheimer's disease.Neuroscience. 2015 Nov 19;309:51-67. doi: 10.1016/j.neuroscience.2015.03.006. Epub 2015 Mar 12. Neuroscience. 2015. PMID: 25772787 Free PMC article. Review.
References
-
- Azizeh BY, Head E, Ibrahim MA, Torp R, Tenner AJ, Kim RC, et al. Molecular dating of senile plaques in the brains of individuals with Down syndrome and in aged dogs. Exp Neurol. 2000;163:111–22. - PubMed
-
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. - PubMed
-
- Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

