Bimodal high-affinity association of Brd4 with murine leukemia virus integrase and mononucleosomes
- PMID: 24520112
- PMCID: PMC4005663
- DOI: 10.1093/nar/gku135
Bimodal high-affinity association of Brd4 with murine leukemia virus integrase and mononucleosomes
Abstract
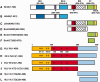
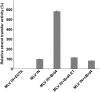
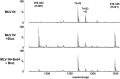
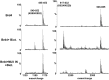
The importance of understanding the molecular mechanisms of murine leukemia virus (MLV) integration into host chromatin is highlighted by the development of MLV-based vectors for human gene-therapy. We have recently identified BET proteins (Brd2, 3 and 4) as the main cellular binding partners of MLV integrase (IN) and demonstrated their significance for effective MLV integration at transcription start sites. Here we show that recombinant Brd4, a representative of the three BET proteins, establishes complementary high-affinity interactions with MLV IN and mononucleosomes (MNs). Brd4(1-720) but not its N- or C-terminal fragments effectively stimulate MLV IN strand transfer activities in vitro. Mass spectrometry- and NMR-based approaches have enabled us to map key interacting interfaces between the C-terminal domain of BRD4 and the C-terminal tail of MLV IN. Additionally, the N-terminal fragment of Brd4 binds to both DNA and acetylated histone peptides, allowing it to bind tightly to MNs. Comparative analyses of the distributions of various histone marks along chromatin revealed significant positive correlations between H3- and H4-acetylated histones, BET protein-binding sites and MLV-integration sites. Our findings reveal a bimodal mechanism for BET protein-mediated MLV integration into select chromatin locations.
Figures




Similar articles
-
BET proteins promote efficient murine leukemia virus integration at transcription start sites.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):12036-41. doi: 10.1073/pnas.1307157110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818621 Free PMC article.
-
Bromo- and extraterminal domain chromatin regulators serve as cofactors for murine leukemia virus integration.J Virol. 2013 Dec;87(23):12721-36. doi: 10.1128/JVI.01942-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049186 Free PMC article.
-
Structure of the Brd4 ET domain bound to a C-terminal motif from γ-retroviral integrases reveals a conserved mechanism of interaction.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):2086-91. doi: 10.1073/pnas.1516813113. Epub 2016 Feb 8. Proc Natl Acad Sci U S A. 2016. PMID: 26858406 Free PMC article.
-
BRD4 in physiology and pathology: ''BET'' on its partners.Bioessays. 2021 Dec;43(12):e2100180. doi: 10.1002/bies.202100180. Epub 2021 Oct 26. Bioessays. 2021. PMID: 34697817 Review.
-
WHAT do viruses BET on?Front Biosci (Landmark Ed). 2010 Jan 1;15(2):537-49. doi: 10.2741/3632. Front Biosci (Landmark Ed). 2010. PMID: 20036832 Review.
Cited by
-
Retroviral DNA Integration.Chem Rev. 2016 Oct 26;116(20):12730-12757. doi: 10.1021/acs.chemrev.6b00125. Epub 2016 May 20. Chem Rev. 2016. PMID: 27198982 Free PMC article. Review.
-
Structure and function of retroviral integrase.Nat Rev Microbiol. 2022 Jan;20(1):20-34. doi: 10.1038/s41579-021-00586-9. Epub 2021 Jul 9. Nat Rev Microbiol. 2022. PMID: 34244677 Free PMC article. Review.
-
The FACT Complex Promotes Avian Leukosis Virus DNA Integration.J Virol. 2017 Mar 13;91(7):e00082-17. doi: 10.1128/JVI.00082-17. Print 2017 Apr 1. J Virol. 2017. PMID: 28122976 Free PMC article.
-
Rationally Designed ACE2-Derived Peptides Inhibit SARS-CoV-2.Bioconjug Chem. 2021 Jan 20;32(1):215-223. doi: 10.1021/acs.bioconjchem.0c00664. Epub 2020 Dec 24. Bioconjug Chem. 2021. PMID: 33356169 Free PMC article.
-
Specialized DNA Structures Act as Genomic Beacons for Integration by Evolutionarily Diverse Retroviruses.Viruses. 2023 Feb 7;15(2):465. doi: 10.3390/v15020465. Viruses. 2023. PMID: 36851678 Free PMC article.
References
-
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. - PubMed
-
- Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. - PubMed
-
- Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 2003;278:372–381. - PubMed
-
- Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P, Ecker JR, Bushman F. A role for LEDGF/p75 in targeting HIV DNA integration. Nat. Med. 2005;11:1287–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

