Plasticity of both planar cell polarity and cell identity during the development of Drosophila
- PMID: 24520160
- PMCID: PMC3918708
- DOI: 10.7554/eLife.01569
Plasticity of both planar cell polarity and cell identity during the development of Drosophila
Abstract
Drosophila has helped us understand the genetic mechanisms of pattern formation. Particularly useful have been those organs in which different cell identities and polarities are displayed cell by cell in the cuticle and epidermis (Lawrence, 1992; Bejsovec and Wieschaus, 1993; Freeman, 1997). Here we use the pattern of larval denticles and muscle attachments and ask how this pattern is maintained and renewed over the larval moult cycles. During larval growth each epidermal cell increases manyfold in size but neither divides nor dies. We follow individuals from moult to moult, tracking marked cells and find that, as cells are repositioned and alter their neighbours, their identities change to compensate and the pattern is conserved. Single cells adopting a new fate may even acquire a new polarity: an identified cell that makes a forward-pointing denticle in the first larval stage may make a backward-pointing denticle in the second and third larval stages. DOI: http://dx.doi.org/10.7554/eLife.01569.001.
Keywords: Drosophila; convergent extension; denticles; pattern; planar cell polarity.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
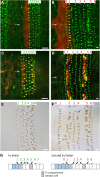
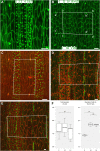

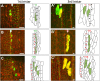
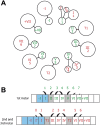

Similar articles
-
Cell shape and epithelial patterning in the Drosophila embryonic epidermis.Fly (Austin). 2009 Jul-Sep;3(3):185-91. doi: 10.4161/fly.3.3.9138. Epub 2009 Jul 27. Fly (Austin). 2009. PMID: 19625773
-
Lamellipodia-based migrations of larval epithelial cells are required for normal closure of the adult epidermis of Drosophila.Dev Biol. 2012 Mar 1;363(1):179-90. doi: 10.1016/j.ydbio.2011.12.033. Epub 2011 Dec 29. Dev Biol. 2012. PMID: 22230614 Free PMC article.
-
Planar cell polarity: the orientation of larval denticles in Drosophila appears to depend on gradients of Dachsous and Fat.Development. 2010 Oct;137(20):3411-5. doi: 10.1242/dev.047126. Epub 2010 Sep 8. Development. 2010. PMID: 20826534 Free PMC article.
-
Forces directing germ-band extension in Drosophila embryos.Mech Dev. 2017 Apr;144(Pt A):11-22. doi: 10.1016/j.mod.2016.12.001. Epub 2016 Dec 22. Mech Dev. 2017. PMID: 28013027 Review.
-
Conservation and divergence in molecular mechanisms of axis formation.Annu Rev Genet. 2001;35:407-37. doi: 10.1146/annurev.genet.35.102401.090832. Annu Rev Genet. 2001. PMID: 11700289 Review.
Cited by
-
A mathematical framework for understanding the spontaneous emergence of complexity applicable to growing multicellular systems.PLoS Comput Biol. 2024 Jun 5;20(6):e1011882. doi: 10.1371/journal.pcbi.1011882. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38838038 Free PMC article.
-
A synthetic planar cell polarity system reveals localized feedback on Fat4-Ds1 complexes.Elife. 2017 Aug 18;6:e24820. doi: 10.7554/eLife.24820. Elife. 2017. PMID: 28826487 Free PMC article.
-
Planar cell polarity: the Dachsous/Fat system contributes differently to the embryonic and larval stages of Drosophila.Biol Open. 2016 Apr 15;5(4):397-408. doi: 10.1242/bio.017152. Biol Open. 2016. PMID: 26935392 Free PMC article.
-
Regions within a single epidermal cell of Drosophila can be planar polarised independently.Elife. 2015 Feb 11;4:e06303. doi: 10.7554/eLife.06303. Elife. 2015. PMID: 25671242 Free PMC article.
-
An anatomical atlas of Drosophila melanogaster-the wild-type.Genetics. 2024 Oct 7;228(2):iyae129. doi: 10.1093/genetics/iyae129. Genetics. 2024. PMID: 39240573 Free PMC article.
References
-
- Alexandre C, Lecourtois M, Vincent J. 1999. Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development 126:5689–5698 - PubMed
-
- Bate M, Martínez Arias A. 1993. The Development of Drosophila melanogaster. 2 Plainview, NY: Cold Spring Harbor Laboratory Press
-
- Becker S, Pasca G, Strumpf D, Min L, Volk T. 1997. Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development 124:2615–2622 - PubMed
-
- Bejsovec A, Wieschaus E. 1993. Segment polarity gene interactions modulate epidermal patterning in Drosophila embryos. Development 119:501–517 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

