Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations
- PMID: 24520174
- PMCID: PMC3948265
- DOI: 10.1073/pnas.1314118111
Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations
Abstract
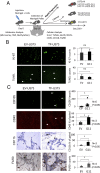
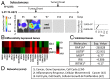
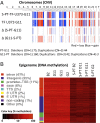
The coagulation system links immediate (hemostatic) and late (inflammatory, angiogenic) tissue responses to injury, a continuum that often is subverted in cancer. Here we provide evidence that tumor dormancy is influenced by tissue factor (TF), the cancer cell-associated initiator of the coagulation system and a signaling receptor. Thus, indolent human glioma cells deficient for TF remain viable but permanently dormant at the injection site for nearly a year, whereas the expression of TF leads to a step-wise transition to latent and overt tumor growth phases, a process that is preceded by recruitment of vascular (CD105(+)) and myeloid (CD11b(+) and F4/80(+)) cells. Importantly, the microenvironment orchestrated by TF expression drives permanent changes in the phenotype, gene-expression profile, DNA copy number, and DNA methylation state of the tumor cells that escape from dormancy. We postulate that procoagulant events in the tissue microenvironment (niche) may affect the fate of occult tumor cells, including their biological and genetic progression to initiate a full-blown malignancy.
Keywords: angiogenesis; brain tumor; clotting; macrophages; oncogenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427(6977):787. - PubMed
-
- Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294(2):139–146. - PubMed
-
- Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328(17):1237–1243. - PubMed
-
- Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10(12):871–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

