Travoprost with sofZia® preservative system lowered intraocular pressure of Japanese normal tension glaucoma with minimal side effects
- PMID: 24520191
- PMCID: PMC3917943
- DOI: 10.2147/OPTH.S57640
Travoprost with sofZia® preservative system lowered intraocular pressure of Japanese normal tension glaucoma with minimal side effects
Abstract
Background: This study aimed to evaluate the effect of travoprost with sofZia® preservative system for lowering the intraocular pressure (IOP) of Japanese normal tension glaucoma (NTG) patients.
Methods: In this prospective, multicenter, open-label study, Japanese NTG patients with baseline IOPs <20 mmHg were enrolled after three consecutive time measurements taken at screening and baseline visits. Travoprost with sofZia® was instilled once daily. Lowering effect on IOP, conjunctival hyperemia, superficial punctate keratopathy, and adverse events were examined at week 4, 8, and 12 after drug instillation.
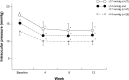
Results: One-hundred and three of the 107 enrolled patients (baseline IOP =15.2±2.0 mmHg [mean ± standard deviation]) completed the study. The mean IOP value as well as percent reduction was significantly reduced at each visit after travoprost with sofZia® initiation (P<0.0001). The conjunctival hyperemia score was 1 or less throughout the study, though it increased significantly over time. No significant change was observed in superficial punctate keratopathy. The cumulative incidence of side effects such as eyelash changes, eyelid pigmentation, and deepening of the upper lid was 47.6%, 27.2%, and 16.5%, respectively.
Conclusion: Travoprost preserved with sofZia® effectively lowered the IOP of Japanese NTG patients. It was well tolerated with few discontinuations due to adverse events.
Keywords: normal tension glaucoma (NTG); prostaglandin analogue; travoprost with sofZia® preservative system.
Figures



References
-
- Netland PA, Landry T, Sullivan EK, et al. Travoprost Study Group Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132(4):472–484. - PubMed
-
- Lewis RA, Katz G, Weiss MJ, et al. Travoprost BAC-free Group Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007;16(1):98–103. - PubMed
-
- Iwase A, Suzuki Y, Araie M, et al. Tajimi Study Group, Japan Glaucoma Society The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111(9):1641–1648. - PubMed
-
- Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: The Beaver Dam Eye Study. Ophthalmology. 1992;99(10):1499–1504. - PubMed
-
- Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998;105(2):209–215. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

