Hepatic glucose intolerance precedes hepatic steatosis in the male aromatase knockout (ArKO) mouse
- PMID: 24520329
- PMCID: PMC3919708
- DOI: 10.1371/journal.pone.0087230
Hepatic glucose intolerance precedes hepatic steatosis in the male aromatase knockout (ArKO) mouse
Abstract
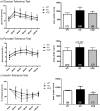
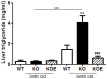
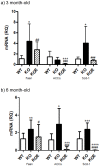
Estrogens are known to play a role in modulating metabolic processes within the body. The Aromatase knockout (ArKO) mice have been shown to harbor factors of Metabolic syndrome with central adiposity, hyperinsulinemia and male-specific hepatic steatosis. To determine the effects of estrogen ablation and subsequent replacement in males on whole body glucose metabolism, three- and six-month-old male ArKO mice were subjected to whole body glucose, insulin and pyruvate tolerance tests and analyzed for ensuing metabolic changes in liver, adipose tissue, and skeletal muscle. Estrogen-deficient male ArKO mice showed increased gonadal adiposity which was significantly reduced upon 17β-estradiol (E2) treatment. Concurrently, elevated ArKO serum leptin levels were significantly reduced upon E2 treatment and lowered serum adiponectin levels were restored to wild type levels. Three-month-old male ArKO mice were hyperglycemic, and both glucose and pyruvate intolerant. These phenotypes continued through to 6 months of age, highlighting a loss of glycemic control. ArKO livers displayed changes in gluconeogenic enzyme expression, and in insulin signaling pathways upon E2 treatment. Liver triglycerides were increased in the ArKO males only after 6 months of age, which could be reversed by E2 treatment. No differences were observed in insulin-stimulated ex vivo muscle glucose uptake nor changes in ArKO adipose tissue and muscle insulin signaling pathways. Therefore, we conclude that male ArKO mice develop hepatic glucose intolerance by the age of 3 months which precedes the sex-specific development of hepatic steatosis. This can be reversed upon the administration of exogenous E2.
Conflict of interest statement
Figures




Similar articles
-
Effects of Estrogens on Adipokines and Glucose Homeostasis in Female Aromatase Knockout Mice.PLoS One. 2015 Aug 28;10(8):e0136143. doi: 10.1371/journal.pone.0136143. eCollection 2015. PLoS One. 2015. PMID: 26317527 Free PMC article.
-
Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse.Endocrinology. 2004 Apr;145(4):1842-8. doi: 10.1210/en.2003-1369. Epub 2003 Dec 18. Endocrinology. 2004. PMID: 14684602
-
Sexual dimorphism in the glucose homeostasis phenotype of the Aromatase Knockout (ArKO) mice.J Steroid Biochem Mol Biol. 2017 Jun;170:39-48. doi: 10.1016/j.jsbmb.2016.05.013. Epub 2016 Jun 25. J Steroid Biochem Mol Biol. 2017. PMID: 27353462 Review.
-
A selective estrogen receptor α agonist ameliorates hepatic steatosis in the male aromatase knockout mouse.J Endocrinol. 2011 Sep;210(3):323-34. doi: 10.1530/JOE-10-0462. Epub 2011 Jun 24. J Endocrinol. 2011. PMID: 21705395
-
Effect of estrogen deficiency in the male: the ArKO mouse model.Mol Cell Endocrinol. 2002 Jul 31;193(1-2):7-12. doi: 10.1016/s0303-7207(02)00090-4. Mol Cell Endocrinol. 2002. PMID: 12160996 Review.
Cited by
-
Treatment with Soluble Activin Type IIB Receptor Ameliorates Ovariectomy-Induced Bone Loss and Fat Gain in Mice.Calcif Tissue Int. 2022 Apr;110(4):504-517. doi: 10.1007/s00223-021-00934-0. Epub 2022 Jan 13. Calcif Tissue Int. 2022. PMID: 35024891 Free PMC article.
-
Estrogens and Body Weight Regulation in Men.Adv Exp Med Biol. 2017;1043:285-313. doi: 10.1007/978-3-319-70178-3_14. Adv Exp Med Biol. 2017. PMID: 29224100 Free PMC article. Review.
-
Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice.Am J Physiol Endocrinol Metab. 2017 Oct 1;313(4):E450-E462. doi: 10.1152/ajpendo.00093.2017. Epub 2017 Jun 27. Am J Physiol Endocrinol Metab. 2017. PMID: 28655716 Free PMC article.
-
Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology.Cells. 2021 Sep 21;10(9):2502. doi: 10.3390/cells10092502. Cells. 2021. PMID: 34572151 Free PMC article. Review.
-
Lack of 17β-estradiol reduces sensitivity to insulin in the liver and muscle of male mice.Heliyon. 2018 Sep 11;4(9):e00772. doi: 10.1016/j.heliyon.2018.e00772. eCollection 2018 Sep. Heliyon. 2018. PMID: 30211334 Free PMC article.
References
-
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, et al. (2004) Dysmetabolic Syndrome in a Man with a Novel Mutation of the Aromatase Gene: Effects of Testosterone, Alendronate, and Estradiol Treatment. J Clin Endocrinol Metab 89: 61–70. - PubMed
-
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K (1995) Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80: 3689–3698. - PubMed
-
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, et al. (1997) Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337: 91–95. - PubMed
-
- Herrmann BL, Janssen OE, Hahn S, Broecker-Preuss M, Mann K (2005) Effects of estrogen replacement therapy on bone and glucose metabolism in a male with congenital aromatase deficiency. Horm Metab Res 37: 178–183. - PubMed
-
- Jones ME, Boon WC, Proietto J, Simpson ER (2006) Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17: 55–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

