Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia
- PMID: 24520391
- PMCID: PMC3919779
- DOI: 10.1371/journal.pone.0088511
Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia
Abstract
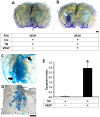
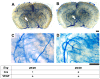
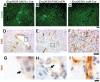
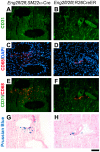
Endoglin (ENG) is a causative gene of type 1 hereditary hemorrhagic telangiectasia (HHT1). HHT1 patients have a higher prevalence of brain arteriovenous malformation (AVM) than the general population and patients with other HHT subtypes. The pathogenesis of brain AVM in HHT1 patients is currently unknown and no specific medical therapy is available to treat patients. Proper animal models are crucial for identifying the underlying mechanisms for brain AVM development and for testing new therapies. However, creating HHT1 brain AVM models has been quite challenging because of difficulties related to deleting Eng-floxed sequence in Eng(2fl/2fl) mice. To create an HHT1 brain AVM mouse model, we used several Cre transgenic mouse lines to delete Eng in different cell-types in Eng(2fl/2fl) mice: R26CreER (all cell types after tamoxifen treatment), SM22α-Cre (smooth muscle and endothelial cell) and LysM-Cre (lysozyme M-positive macrophage). An adeno-associated viral vector expressing vascular endothelial growth factor (AAV-VEGF) was injected into the brain to induce focal angiogenesis. We found that SM22α-Cre-mediated Eng deletion in the embryo caused AVMs in the postnatal brain, spinal cord, and intestines. Induction of Eng deletion in adult mice using R26CreER plus local VEGF stimulation induced the brain AVM phenotype. In both models, Eng-null endothelial cells were detected in the brain AVM lesions, and formed mosaicism with wildtype endothelial cells. However, LysM-Cre-mediated Eng deletion in the embryo did not cause AVM in the postnatal brain even after VEGF stimulation. In this study, we report two novel HHT1 brain AVM models that mimic many phenotypes of human brain AVM and can thus be used for studying brain AVM pathogenesis and testing new therapies. Further, our data indicate that macrophage Eng deletion is insufficient and that endothelial Eng homozygous deletion is required for HHT1 brain AVM development.
Conflict of interest statement
Figures

References
-
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, et al. (1994) Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8: 345–351. - PubMed
-
- Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, et al. (2000) Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 91: 66–67. - PubMed
-
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, et al. (1996) Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13: 189–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

