Endocannabinoid modulation of cortical up-states and NREM sleep
- PMID: 24520411
- PMCID: PMC3919802
- DOI: 10.1371/journal.pone.0088672
Endocannabinoid modulation of cortical up-states and NREM sleep
Abstract
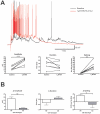
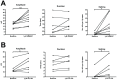
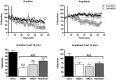
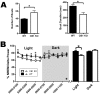
Up-/down-state transitions are a form of network activity observed when sensory input into the cortex is diminished such as during non-REM sleep. Up-states emerge from coordinated signaling between glutamatergic and GABAergic synapses and are modulated by systems that affect the balance between inhibition and excitation. We hypothesized that the endocannabinoid (EC) system, a neuromodulatory system intrinsic to the cortical microcircuitry, is an important regulator of up-states and sleep. To test this hypothesis, up-states were recorded from layer V/VI pyramidal neurons in organotypic cultures of wild-type or CB1R knockout (KO) mouse prefrontal cortex. Activation of the cannabinoid 1 receptor (CB1) with exogenous agonists or by blocking metabolism of endocannabinoids, anandamide or 2-arachidonoyl glycerol, increased up-state amplitude and facilitated action potential discharge during up-states. The CB1 agonist also produced a layer II/III-selective reduction in synaptic GABAergic signaling that may underlie its effects on up-state amplitude and spiking. Application of CB1 antagonists revealed that an endogenous EC tone regulates up-state duration. Paradoxically, the duration of up-states in CB1 KO cultures was increased suggesting that chronic absence of EC signaling alters cortical activity. Consistent with increased cortical excitability, CB1 KO mice exhibited increased wakefulness as a result of reduced NREM sleep and NREM bout duration. Under baseline conditions, NREM delta (0.5-4 Hz) power was not different in CB1 KO mice, but during recovery from forced sleep deprivation, KO mice had reduced NREM delta power and increased sleep fragmentation. Overall, these findings demonstrate that the EC system actively regulates cortical up-states and important features of NREM sleep such as its duration and low frequency cortical oscillations.
Conflict of interest statement
Figures







Similar articles
-
Endocannabinoid Signaling Regulates Sleep Stability.PLoS One. 2016 Mar 31;11(3):e0152473. doi: 10.1371/journal.pone.0152473. eCollection 2016. PLoS One. 2016. PMID: 27031992 Free PMC article.
-
Increased Contextual Fear Conditioning in iNOS Knockout Mice: Additional Evidence for the Involvement of Nitric Oxide in Stress-Related Disorders and Contribution of the Endocannabinoid System.Int J Neuropsychopharmacol. 2015 Jan 24;18(8):pyv005. doi: 10.1093/ijnp/pyv005. Int J Neuropsychopharmacol. 2015. PMID: 25618404 Free PMC article.
-
Multiple Forms of Endocannabinoid and Endovanilloid Signaling Regulate the Tonic Control of GABA Release.J Neurosci. 2015 Jul 8;35(27):10039-57. doi: 10.1523/JNEUROSCI.4112-14.2015. J Neurosci. 2015. PMID: 26157003 Free PMC article.
-
Endocannabinoid-mediated retrograde modulation of synaptic transmission.Curr Opin Neurobiol. 2014 Dec;29:1-8. doi: 10.1016/j.conb.2014.03.017. Epub 2014 Apr 18. Curr Opin Neurobiol. 2014. PMID: 24747340 Review.
-
Why do cannabinoid receptors have more than one endogenous ligand?Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3216-28. doi: 10.1098/rstb.2011.0382. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108541 Free PMC article. Review.
Cited by
-
Dose-dependent cannabis use, depressive symptoms, and FAAH genotype predict sleep quality in emerging adults: a pilot study.Am J Drug Alcohol Abuse. 2016 Jul;42(4):431-40. doi: 10.3109/00952990.2016.1141913. Epub 2016 Apr 13. Am J Drug Alcohol Abuse. 2016. PMID: 27074158 Free PMC article.
-
Tonic endocannabinoid signaling supports sleep through development in both sexes.Sleep. 2022 Aug 11;45(8):zsac083. doi: 10.1093/sleep/zsac083. Sleep. 2022. PMID: 35395682 Free PMC article.
-
Role of the Endocannabinoid System in the Pathophysiology of Schizophrenia.Mol Neurobiol. 2017 Jan;54(1):768-778. doi: 10.1007/s12035-016-9697-5. Epub 2016 Jan 15. Mol Neurobiol. 2017. PMID: 26768595 Review.
-
Additive effect of 5-HT2C and CB1 receptor blockade on the regulation of sleep-wake cycle.BMC Neurosci. 2019 Mar 20;20(1):14. doi: 10.1186/s12868-019-0495-7. BMC Neurosci. 2019. PMID: 30894126 Free PMC article.
-
Endocannabinoid Signaling Regulates Sleep Stability.PLoS One. 2016 Mar 31;11(3):e0152473. doi: 10.1371/journal.pone.0152473. eCollection 2016. PLoS One. 2016. PMID: 27031992 Free PMC article.
References
-
- Amzica F, Steriade M (1998) Electrophysiological correlates of sleep delta waves. Electroencephalography and Clinical Neurophysiology 107: 69–83. - PubMed
-
- Lambe EK, Aghajanian GK (2006) Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology 31: 1682–1689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

