Anti-tumor effects of novel 5-O-acyl plumbagins based on the inhibition of mammalian DNA replicative polymerase activity
- PMID: 24520419
- PMCID: PMC3919815
- DOI: 10.1371/journal.pone.0088736
Anti-tumor effects of novel 5-O-acyl plumbagins based on the inhibition of mammalian DNA replicative polymerase activity
Abstract
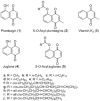
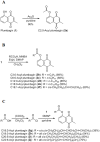
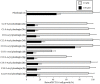
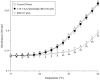
We previously found that vitamin K3 (menadione, 2-methyl-1,4-naphthoquinone) inhibits the activity of human mitochondrial DNA polymerase γ (pol γ). In this study, we focused on plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), and chemically synthesized novel plumbagins conjugated with C2:0 to C22:6 fatty acids (5-O-acyl plumbagins). These chemically modified plumbagins enhanced mammalian pol inhibition and their cytotoxic activity. Plumbagin conjugated with chains consisting of more than C18-unsaturated fatty acids strongly inhibited the activities of calf pol α and human pol γ. Plumbagin conjugated with oleic acid (C18:1-acyl plumbagin) showed the strongest suppression of human colon carcinoma (HCT116) cell proliferation among the ten synthesized 5-O-acyl plumbagins. The inhibitory activity on pol α, a DNA replicative pol, by these compounds showed high correlation with their cancer cell proliferation suppressive activity. C18:1-Acyl plumbagin selectively inhibited the activities of mammalian pol species, but did not influence the activities of other pols and DNA metabolic enzymes tested. This compound inhibited the proliferation of various human cancer cell lines, and was the cytotoxic inhibitor showing strongest inhibition towards HT-29 colon cancer cells (LD50 = 2.9 µM) among the nine cell lines tested. In an in vivo anti-tumor assay conducted on nude mice bearing solid tumors of HT-29 cells, C18:1-acyl plumbagin was shown to be a promising tumor suppressor. These data indicate that novel 5-O-acyl plumbagins act as anti-cancer agents based on mammalian DNA replicative pol α inhibition. Moreover, the results suggest that acylation of plumbagin is an effective chemical modification to improve the anti-cancer activity of vitamin K3 derivatives, such as plumbagin.
Conflict of interest statement
Figures




References
-
- Sakaguchi K, Sugawara F, Mizushina Y (2002) Inhibitors of eukaryotic DNA polymerases. Seikagaku 74: 244–251. - PubMed
-
- Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer 3: 768–780. - PubMed
-
- Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 134: 3479S–3485S. - PubMed
-
- Kornberg K, Baker TA (1992) DNA replication. FreemanW.D. and Co. New York, University Science Books. 197–225p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

