Effectiveness of milk whey protein-based ready-to-use therapeutic food in treatment of severe acute malnutrition in Malawian under-5 children: a randomised, double-blind, controlled non-inferiority clinical trial
- PMID: 24521353
- PMCID: PMC6860310
- DOI: 10.1111/mcn.12112
Effectiveness of milk whey protein-based ready-to-use therapeutic food in treatment of severe acute malnutrition in Malawian under-5 children: a randomised, double-blind, controlled non-inferiority clinical trial
Abstract
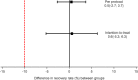
The cost of ready-to-use therapeutic food (RUTF) used in community-based management of acute malnutrition has been a major obstacle to the scale up of this important child survival strategy. The current standard recipe for RUTF [peanut-based RUTF (P-RUTF)] is made from peanut paste, milk powder, oil, sugar, and minerals and vitamins. Milk powder forms about 30% of the ingredients and may represent over half the cost of the final product. The quality of whey protein concentrates 34% (WPC34) is similar to that of dried skimmed milk (DSM) used in the standard recipe and can be 25-33% cheaper. This blinded, parallel group, randomised, controlled non-inferiority clinical trial tested the effectiveness in treating severe acute malnutrition (SAM) of a new RUTF formulation WPC-RUTF in which WPC34 was used to replace DSM. Average weight gain (non-inferiority margin Δ = -1.2 g kg(-1) day(-1) ) and recovery rate (Δ = -10%) were the primary outcomes, and length of stay (LOS) was the secondary outcome (Δ = +14 days). Both per-protocol (PP) and intention-to-treat (ITT) analyses showed that WPC-RUTF was not inferior to P-RUTF for recovery rate [difference and its 95% confidence interval (CI) of 0.5% (95% CI -2.7, 3.7) in PP analysis and 0.6% (95% CI -5.2, 6.3) in ITT analysis] for average weight gain [0.2 (-0.5; 0.9) for both analyses] and LOS [-1.6 days (95% CI, -4.6, 1.4 days) in PP analysis and -1.9 days (95% CI, -4.6, 0.8 days) for ITT analysis]. In conclusion, whey protein-based RUTF is an effective cheaper alternative to the standard milk-based RUTF for the treatment of SAM.
Keywords: community-based; malnutrition; randomised controlled trial; therapeutic feeding; undernutrition; whey protein.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
VO was an employee of Valid Nutrition. SC is the unpaid director of Valid Nutrition. Valid International is the sister company of Valid Nutrition that promotes the development and promotion of RUTF. Clinton Foundation and USDEC had no say on the design, implementation and interpretation of the results.
Figures


References
-
- Ali E., Zachariah R., Shams Z., Manzi M., Akter T., Alders P., Allaouna M., Delchevalerie P. & Harries A.D. (2013) Peanut‐based ready‐to‐use therapeutic food: how acceptable and tolerated is it among malnourished pregnant and lactating women in Bangladesh? Maternal and Child Nutrition. doi: 10.1111/mcn.12050 - DOI - PMC - PubMed
-
- Ashworth A. (2006) Efficacy and effectiveness of community‐based treatment of severe malnutrition. Food and Nutrition Bulletin 27 (Suppl. 3), S24–S48. - PubMed
-
- Bahwere P., Piwoz E., Joshua M.C., Sadler K., Grobler‐Tanner C.H., Guerrero S. & Collins S. (2008) Uptake of HIV testing and outcomes within a Community‐based Therapeutic Care (CTC) programme to treat severe acute malnutrition in Malawi: a descriptive study. BMC Infectious Diseases 8, 106. - PMC - PubMed
-
- Barth C.A. & Behnke U. (1997) Nutritional physiology of whey and whey components. Die Nahrung 41, 2–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

