Sex differences in responses to antiretroviral treatment in South African HIV-infected children on ritonavir-boosted lopinavir- and nevirapine-based treatment
- PMID: 24521425
- PMCID: PMC3927631
- DOI: 10.1186/1471-2431-14-39
Sex differences in responses to antiretroviral treatment in South African HIV-infected children on ritonavir-boosted lopinavir- and nevirapine-based treatment
Abstract
Background: While studies of HIV-infected adults on antiretroviral treatment (ART) report no sex differences in immune recovery and virologic response but more ART-associated complications in women, sex differences in disease progression and response to ART among children have not been well assessed. The objective of this study was to evaluate for sex differences in response to ART in South African HIV-infected children who were randomized to continue ritonavir-boosted lopinavir (LPV/r)-based ART or switch to nevirapine-based ART.
Methods: ART outcomes in HIV-infected boys and girls in Johannesburg, South Africa from 2005-2010 were compared. Children initiated ritonavir-boosted lopinavir (LPV/r)-based ART before 24 months of age and were randomized to remain on LPV/r or switch to nevirapine-based ART after achieving viral suppression. Children were followed for 76 weeks post-randomization and then long-term follow up continued for a minimum of 99 weeks and maximum of 245 weeks after randomization. Viral load, CD4 count, lipids, anthropometrics, drug concentrations, and adherence were measured at regular intervals. Outcomes were compared between sexes within treatment strata.
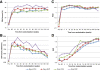
Results: A total of 323 children (median age 8.8 months, IQR 5.1-13.5), including 168 boys and 155 girls, initiated LPV/r-based ART and 195 children were randomized. No sex differences in risk of virological failure (confirmed viral load >1000 copies/mL) by 156 weeks post-randomization were observed within either treatment group. Girls switched to nevirapine had more robust CD4 count improvement relative to boys in this group through 112 weeks post-randomization. In addition, girls remaining on LPV/r had higher plasma concentrations of ritonavir than boys during post-randomization visits. After a mean of 3.4 years post-randomization, girls remaining on LPV/r also had a higher total cholesterol:HDL ratio and lower mean HDL than boys on LPV/r.
Conclusions: Sex differences are noted in treated HIV-infected children even at a young age, and appear to depend on treatment regimen. Future studies are warranted to determine biological mechanisms and clinical significance of these differences.
Trial registration: ClinicalTrials.gov Identifier: NCT00117728.
Figures
References
-
- Soon GG, Min M, Struble KA, Chan-Tack KM, Hammerstrom T, Qi K, Zhou S, Bhore R, Murray JS, Birnkrant DB. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008) AIDS Patient Care STDS. 2012;26(8):444–453. doi: 10.1089/apc.2011.0278. - DOI - PubMed
-
- Moore AL, Kirk O, Johnson AM, Katlama C, Blaxhult A, Dietrich M, Colebunders R, Chiesi A, Lungren JD, Phillips AN. Virologic, immunologic, and clinical response to highly active antiretroviral therapy: the gender issue revisited. J Acquir Immune Defic Syndr. 2003;32(4):452–461. doi: 10.1097/00126334-200304010-00017. - DOI - PubMed
-
- Ofotokun I, Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11(2):55–59. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

