Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma
- PMID: 24521523
- PMCID: PMC4317809
- DOI: 10.1111/cas.12368
Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma
Abstract
GC33 is a humanized mAb against human glypican-3 (GPC3). In the first-in-human study carried out in the USA, GC33 was well tolerated and showed preliminary antitumor activity in patients with advanced hepatocellular carcinoma. This study aimed to assess the safety, tolerability, and pharmacokinetic characteristics of GC33 in Japanese patients with advanced hepatocellular carcinoma. The study design was a conventional 3 + 3 dose-escalation design to determine the maximum tolerated dose of GC33 given i.v. at 5, 10, or 20 mg/kg weekly. Immunohistochemistry was carried out on tumor biopsies to evaluate GPC3 expression. Thirteen patients were enrolled across the three dose levels, and no patients observed any dose-limiting toxicity up to the highest planned dose of 20 mg/kg. The most common adverse events were decreased lymphocyte count, decreased natural killer cell count, increased C-reactive protein, and pyrexia. Grade 3 adverse events (increased blood pressure, decreased lymphocyte count, and decreased platelet count) were observed in two or more patients. The AUCinf showed a dose-proportional increase from the 5 mg/kg dose group to the 20 mg/kg dose group. The trough concentrations of GC33 appeared to reach a steady state after the fourth to the sixth dose. Seven of the 13 patients showed stable disease, the other six showed progressive disease. Furthermore, three patients showed long-term stable disease of more than 5 months. In conclusion, GC33 given at up to 20 mg/kg weekly was well tolerated in Japanese patients with advanced hepatocellular carcinoma.
Keywords: GC33; Japanese patients; glypican-3; hepatocellular carcinoma; phase I study.
© 2014 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures
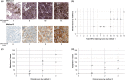
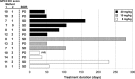
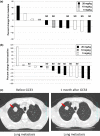
References
-
- Alves RC, Alves D, Guz B, et al. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann Hepatol. 2011;10:21–7. - PubMed
-
- Ferenci P, Fried M, Labrecque D, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239–45. - PubMed
-
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med. 2008;359:378–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

