More potent lipid-lowering effect by rosuvastatin compared with fluvastatin in everolimus-treated renal transplant recipients
- PMID: 24521776
- PMCID: PMC4127422
- DOI: 10.1097/01.TP.0000443225.66960.7e
More potent lipid-lowering effect by rosuvastatin compared with fluvastatin in everolimus-treated renal transplant recipients
Abstract
Background: Dyslipidemia is a risk factor for premature cardiovascular morbidity and mortality in renal transplant recipients (RTRs). Pharmacotherapy with mTOR inhibitors aggravates dyslipidemia, thus necessitating lipid-lowering therapy with fluvastatin, pravastatin, or atorvastatin. These agents may not sufficiently lower lipid levels, and therefore, a more potent agent like rosuvastatin maybe needed.
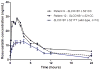
Methods: We have aimed to assess the lipid-lowering effect of rosuvastatin as compared with fluvastatin in RTR receiving everolimus. Safety was assessed as the pharmacokinetic (PK) interaction potential of a rosuvastatin/everolimus combination in RTR. A 12-hour everolimus PK investigation was performed in 12 stable RTR receiving everolimus and fluvastatin (80 mg/d). Patients were then switched to rosuvastatin (20 mg/d), and a follow-up 12/24-hour PK investigation of everolimus/rosuvastatin was performed after 1 month. All other drugs were kept unchanged.
Results: In RTR already receiving fluvastatin, switching to rosuvastatin further decreased LDL cholesterol and total cholesterol by 30.2±12.2% (P<0.01) and 18.2±9.6% (P<0.01), respectively. Everolimus AUC0-12 was not affected by concomitant rosuvastatin treatment, 80.3±21.3 μg*h/L before and 78.5±21.9 μg*h/L after, respectively (P=0.61). Mean rosuvastatin AUC0-24 was 157±61.7 ng*h/mL, approximately threefold higher than reported in the literature for nontransplants. There were no adverse events, and none of the patients had or developed proteinuria.
Conclusion: Rosuvastatin showed a superior lipid-lowering effect compared to fluvastatin in stable RTR receiving everolimus. The combination of everolimus/rosuvastatin seems to be as safe as the everolimus/fluvastatin combination.
Trial registration: ClinicalTrials.gov NCT01524601.
Conflict of interest statement
Figures

References
-
- Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82:603. - PubMed
-
- Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307. - PubMed
-
- Lindholm A, Albrechtsen D, Frodin L, Tufveson G, Persson NH, Lundgren G. Ischemic heart disease - Major cause of death and graft loss after renal transplantation in Scandinavia. Transplantation. 1995;60:451. - PubMed
-
- Arend SM, Mallat MJK, Westendorp RJW, vanderwoude FJ, vanEs LA. Patient survival after renal transplantation; more than 25 years follow-up. Nephrol Dial Transplant. 1997;12:1672. - PubMed
-
- Hjelmesaeth J, Hartmann A, Midtvedt K, Aakhus S, Stenstrom J, Morkrid L, et al. Metabolic cardiovascular syndrome after renal transplantation. Nephrol Dial Transplant. 2001;16:1047. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

