Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors
- PMID: 24521784
- PMCID: PMC3993125
- DOI: 10.1128/CVI.00688-13
Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors
Abstract
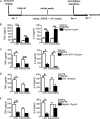
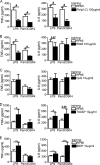
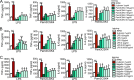
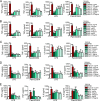
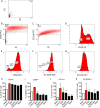
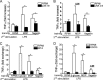
Upon priming with Candida albicans or with the fungal cell wall component β-glucan, monocytes respond with an increased cytokine production upon restimulation, a phenomenon termed "trained immunity." In contrast, the prestimulation of monocytes with lipopolysaccharide has long been known to induce tolerance. Because the vast majority of commensal microorganisms belong to bacterial or viral phyla, we sought to systematically investigate the functional reprogramming of monocytes induced by the stimulation of pattern recognition receptors (PRRs) with various bacterial or viral ligands. Monocytes were functionally programmed for either enhanced (training) or decreased (tolerance) cytokine production, depending on the type and concentration of ligand they encountered. The functional reprogramming of monocytes was also associated with cell shape, granulocity, and cell surface marker modifications. The training effect required p38- and Jun N-terminal protein kinase (JNK)-mediated mitogen-activated protein kinase (MAPK) signaling, with specific signaling patterns directing the functional fate of the cell. The long-term effects on the function of monocytes were mediated by epigenetic events, with both histone methylation and acetylation inhibitors blocking the training effects. In conclusion, our experiments identify the ability of monocytes to acquire adaptive characteristics after prior activation with a wide variety of ligands. Trained immunity and tolerance are two distinct and opposing functional programs induced by the specific microbial ligands engaging the monocytes.
Figures

References
-
- Rheins LA, Karp RD, Butz A. 1978. Humoral Immunity Induced in American Cockroach (Periplaneta americana). Am. Zool. 18:595
-
- Arala-Chaves M, Sequeira T. 2000. Is there any kind of adaptive immunity in invertebrates? Aquaculture 191:247–258. 10.1016/S0044-8486(00)00430-0 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

