Effect of CYP3A4*22, CYP3A5*3, and CYP3A Combined Genotypes on Cyclosporine, Everolimus, and Tacrolimus Pharmacokinetics in Renal Transplantation
- PMID: 24522145
- PMCID: PMC3944116
- DOI: 10.1038/psp.2013.78
Effect of CYP3A4*22, CYP3A5*3, and CYP3A Combined Genotypes on Cyclosporine, Everolimus, and Tacrolimus Pharmacokinetics in Renal Transplantation
Abstract
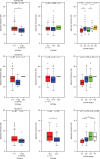
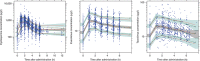
Cyclosporine, everolimus, and tacrolimus are the cornerstone of immunosuppressive therapy in renal transplantation. These drugs are characterized by narrow therapeutic windows, highly variable pharmacokinetics (PK), and metabolism by CYP3A enzymes. Recently, the decreased activity allele, CYP3A4*22, was described as a potential predictive marker for CYP3A4 activity. This study investigated the effect of CYP3A4*22, CYP3A5*3, and CYP3A combined genotypes on cyclosporine, everolimus, and tacrolimus PK in renal transplant patients. CYP3A4*22 carriers showed a significant lower clearance for cyclosporine (-15%), and a trend was observed for everolimus (-7%) and tacrolimus (-16%). Patients carrying at least one CYP3A5*1 allele had 1.5-fold higher tacrolimus clearance compared with noncarriers; however, CYP3A5*3 appeared to be nonpredictive for everolimus and cyclosporine. CYP3A combined genotype did not significantly improve prediction of clearance compared with CYP3A5*3 or CYP3A4*22 alone. These data suggest that dose individualization of cyclosporine, everolimus, or tacrolimus therapy based on CYP3A4*22 is not indicated.CPT: Pharmacometrics Systems Pharmacology (2014); 3, e100; doi:10.1038/psp.2013.78; published online 12 February 2014.
Figures
References
-
- Kuypers D.R., de Jonge H., Naesens M., Lerut E., Verbeke K., Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin. Pharmacol. Ther. 2007;82:711–725. - PubMed
-
- Kovarik J.M., et al. Everolimus Phase 2 Study Group Longitudinal assessment of everolimus in de novo renal transplant recipients over the first post-transplant year: pharmacokinetics, exposure-response relationships, and influence on cyclosporine. Clin. Pharmacol. Ther. 2001;69:48–56. - PubMed
-
- Morse G.D., Holdsworth M.T., Venuto R.C., Gerbasi J., Walshe J.J. Pharmacokinetics and clinical tolerance of intravenous and oral cyclosporine in the immediate postoperative period. Clin. Pharmacol. Ther. 1988;44:654–664. - PubMed
-
- Kirchner G.I., Meier-Wiedenbach I., Manns M.P. Clinical pharmacokinetics of everolimus. Clin. Pharmacokinet. 2004;43:83–95. - PubMed
-
- Jacobsen W., Serkova N., Hausen B., Morris R.E., Benet L.Z., Christians U. Comparison of the in vitro metabolism of the macrolide immunosuppressants sirolimus and RAD. Transplant. Proc. 2001;33:514–515. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

