A cascade of ER exit site assembly that is regulated by p125A and lipid signals
- PMID: 24522181
- PMCID: PMC3986675
- DOI: 10.1242/jcs.138784
A cascade of ER exit site assembly that is regulated by p125A and lipid signals
Abstract
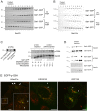
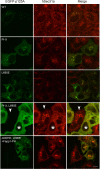
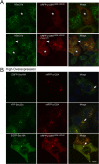
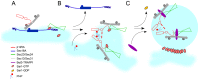
The inner and outer layers of COPII mediate cargo sorting and vesicle biogenesis. Sec16A and p125A (officially known as SEC23IP) proteins interact with both layers to control coat activity, yet the steps directing functional assembly at ER exit sites (ERES) remain undefined. By using temperature blocks, we find that Sec16A is spatially segregated from p125A-COPII-coated ERES prior to ER exit at a step that required p125A. p125A used lipid signals to control ERES assembly. Within p125A, we defined a C-terminal DDHD domain found in phospholipases and PI transfer proteins that recognized PA and phosphatidylinositol phosphates in vitro and was targeted to PI4P-rich membranes in cells. A conserved central SAM domain promoted self-assembly and selective lipid recognition by the DDHD domain. A basic cluster and a hydrophobic interface in the DDHD and SAM domains, respectively, were required for p125A-mediated functional ERES assembly. Lipid recognition by the SAM-DDHD module was used to stabilize membrane association and regulate the spatial segregation of COPII from Sec16A, nucleating the coat at ERES for ER exit.
Keywords: COPII; ERES; Phosphatidylinositol-4-phosphate; Sec23ip; p125A.
Figures




Similar articles
-
p125A (Sec23ip) couples COPII coat assembly with donor-acceptor membrane organization to facilitate tunnel-based traffic.bioRxiv [Preprint]. 2025 May 13:2025.05.07.652703. doi: 10.1101/2025.05.07.652703. bioRxiv. 2025. PMID: 40463098 Free PMC article. Preprint.
-
p125A exists as part of the mammalian Sec13/Sec31 COPII subcomplex to facilitate ER-Golgi transport.J Cell Biol. 2010 Aug 9;190(3):331-45. doi: 10.1083/jcb.201003005. Epub 2010 Aug 2. J Cell Biol. 2010. PMID: 20679433 Free PMC article.
-
Insights into structural and regulatory roles of Sec16 in COPII vesicle formation at ER exit sites.Mol Biol Cell. 2012 Aug;23(15):2930-42. doi: 10.1091/mbc.E12-05-0356. Epub 2012 Jun 6. Mol Biol Cell. 2012. PMID: 22675024 Free PMC article.
-
Vesicle-mediated export from the ER: COPII coat function and regulation.Biochim Biophys Acta. 2013 Nov;1833(11):2464-72. doi: 10.1016/j.bbamcr.2013.02.003. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23419775 Free PMC article. Review.
-
COPII coat assembly and selective export from the endoplasmic reticulum.J Biochem. 2004 Dec;136(6):755-60. doi: 10.1093/jb/mvh184. J Biochem. 2004. PMID: 15671485 Review.
Cited by
-
Lipid transfer proteins and instructive regulation of lipid kinase activities: Implications for inositol lipid signaling and disease.Adv Biol Regul. 2020 Dec;78:100740. doi: 10.1016/j.jbior.2020.100740. Epub 2020 Jul 14. Adv Biol Regul. 2020. PMID: 32992233 Free PMC article. Review.
-
The interface between phosphatidylinositol transfer protein function and phosphoinositide signaling in higher eukaryotes.J Lipid Res. 2019 Feb;60(2):242-268. doi: 10.1194/jlr.R089730. Epub 2018 Nov 30. J Lipid Res. 2019. PMID: 30504233 Free PMC article. Review.
-
Proteomic Screen for Cellular Targets of the Vaccinia Virus F10 Protein Kinase Reveals that Phosphorylation of mDia Regulates Stress Fiber Formation.Mol Cell Proteomics. 2017 Apr;16(4 suppl 1):S124-S143. doi: 10.1074/mcp.M116.065003. Epub 2017 Feb 9. Mol Cell Proteomics. 2017. PMID: 28183815 Free PMC article.
-
Tales of tails in transporters.Open Biol. 2019 Jun 28;9(6):190083. doi: 10.1098/rsob.190083. Epub 2019 Jun 19. Open Biol. 2019. PMID: 31213137 Free PMC article. Review.
-
GRASP55 restricts early-stage autophagy and regulates spatial organization of the early secretory network.Biol Open. 2021 Oct 15;10(10):bio058736. doi: 10.1242/bio.058736. Epub 2021 Oct 12. Biol Open. 2021. PMID: 34533192 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

