α-catenin cytomechanics--role in cadherin-dependent adhesion and mechanotransduction
- PMID: 24522187
- PMCID: PMC3986676
- DOI: 10.1242/jcs.139014
α-catenin cytomechanics--role in cadherin-dependent adhesion and mechanotransduction
Abstract
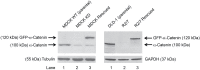
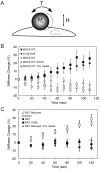
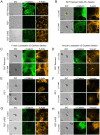
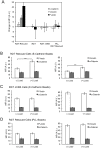
The findings presented here demonstrate the role of α-catenin in cadherin-based adhesion and mechanotransduction in different mechanical contexts. Bead-twisting measurements in conjunction with imaging, and the use of different cell lines and α-catenin mutants reveal that the acute local mechanical manipulation of cadherin bonds triggers vinculin and actin recruitment to cadherin adhesions in an actin- and α-catenin-dependent manner. The modest effect of α-catenin on the two-dimensional binding affinities of cell surface cadherins further suggests that force-activated adhesion strengthening is due to enhanced cadherin-cytoskeletal interactions rather than to α-catenin-dependent affinity modulation. Complementary investigations of cadherin-based rigidity sensing also suggest that, although α-catenin alters traction force generation, it is not the sole regulator of cell contractility on compliant cadherin-coated substrata.
Keywords: Adhesion; Cadherin; Mechanotransduction; α-Catenin.
Figures


References
-
- Bajpai S., Correia J., Feng Y., Figueiredo J., Sun S. X., Longmore G. D., Suriano G., Wirtz D. (2008). alpha-Catenin mediates initial E-cadherin-dependent cell-cell recognition and subsequent bond strengthening. Proc. Natl. Acad. Sci. USA 105, 18331–18336 10.1073/pnas.0806783105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

