Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection
- PMID: 24522913
- PMCID: PMC3993813
- DOI: 10.1128/JVI.03149-13
Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection
Abstract
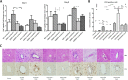
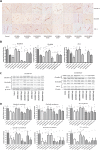
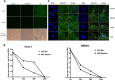
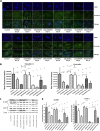
Infection with laboratory-attenuated rabies virus (RABV) enhances blood-brain barrier (BBB) permeability, which has been demonstrated to be an important factor for host survival, since it allows immune effectors to enter the central nervous system (CNS) and clear RABV. To probe the mechanism by which RABV infection enhances BBB permeability, the expression of tight junction (TJ) proteins in the CNS was investigated following intracranial inoculation with laboratory-attenuated or wild-type (wt) RABV. BBB permeability was significantly enhanced in mice infected with laboratory-attenuated, but not wt, RABV. The expression levels of TJ proteins (claudin-5, occludin, and zonula occludens-1) were decreased in mice infected with laboratory-attenuated, but not wt, RABV, suggesting that enhancement of BBB permeability is associated with the reduction of TJ protein expression in RABV infection. RABV neither infects the brain microvascular endothelial cells (BMECs) nor modulates the expression of TJ proteins in BMECs. However, brain extracts prepared from mice infected with laboratory-attenuated, but not wt, RABV reduced TJ protein expression in BMECs. It was found that brain extracts from mice infected with laboratory-attenuated RABV contained significantly higher levels of inflammatory chemokines/cytokines than those from mice infected with wt RABV. Pathway analysis indicates that gamma interferon (IFN-γ) is located in the center of the cytokine network in the RABV-infected mouse brain, and neutralization of IFN-γ reduced both the disruption of BBB permeability in vivo and the downregulation of TJ protein expression in vitro. These findings indicate that the enhancement of BBB permeability and the reduction of TJ protein expression are due not to RABV infection per se but to virus-induced inflammatory chemokines/cytokines.
Importance: Previous studies have shown that infection with only laboratory-attenuated, not wild-type, rabies virus (RABV) enhances blood-brain barrier (BBB) permeability, allowing immune effectors to enter the central nervous system (CNS) and clear RABV from the CNS. This study investigated the mechanism by which RABV infection enhances BBB permeability. It was found that RABV infection enhances BBB permeability by downregulation of tight junction (TJ) protein expression in the brain microvasculature. It was further found that it is not RABV infection per se but the chemokines/cytokines induced by RABV infection that downregulate the expression of TJ proteins and enhance BBB permeability. Blocking some of these cytokines, such as IFN-γ, ameliorated both the disruption of BBB permeability and the downregulation of TJ protein expression. These studies may provide a foundation for developing therapeutics for clinical rabies, such as medication that could be used to enhance BBB permeability.
Figures


Similar articles
-
Lab-Attenuated Rabies Virus Facilitates Opening of the Blood-Brain Barrier by Inducing Matrix Metallopeptidase 8.J Virol. 2022 Sep 14;96(17):e0105022. doi: 10.1128/jvi.01050-22. Epub 2022 Aug 25. J Virol. 2022. PMID: 36005758 Free PMC article.
-
Expression of neuronal CXCL10 induced by rabies virus infection initiates infiltration of inflammatory cells, production of chemokines and cytokines, and enhancement of blood-brain barrier permeability.J Virol. 2015 Jan;89(1):870-6. doi: 10.1128/JVI.02154-14. Epub 2014 Oct 22. J Virol. 2015. PMID: 25339777 Free PMC article.
-
Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection.Virus Res. 2009 Sep;144(1-2):18-26. doi: 10.1016/j.virusres.2009.03.014. Epub 2009 Apr 5. Virus Res. 2009. PMID: 19720239 Free PMC article.
-
The Immune Escape Strategy of Rabies Virus and Its Pathogenicity Mechanisms.Viruses. 2024 Nov 14;16(11):1774. doi: 10.3390/v16111774. Viruses. 2024. PMID: 39599888 Free PMC article. Review.
-
Role of chemokines in rabies pathogenesis and protection.Adv Virus Res. 2011;79:73-89. doi: 10.1016/B978-0-12-387040-7.00005-6. Adv Virus Res. 2011. PMID: 21601043 Free PMC article. Review.
Cited by
-
The Multifaceted Roles of TAM Receptors during Viral Infection.Virol Sin. 2021 Feb;36(1):1-12. doi: 10.1007/s12250-020-00264-9. Epub 2020 Jul 27. Virol Sin. 2021. PMID: 32720213 Free PMC article.
-
SARS-CoV-2 productively infects human brain microvascular endothelial cells.J Neuroinflammation. 2022 Jun 15;19(1):149. doi: 10.1186/s12974-022-02514-x. J Neuroinflammation. 2022. PMID: 35705998 Free PMC article.
-
Knowns and unknowns of TiLV-associated neuronal disease.Virulence. 2024 Dec;15(1):2329568. doi: 10.1080/21505594.2024.2329568. Epub 2024 Mar 31. Virulence. 2024. PMID: 38555518 Free PMC article. Review.
-
Exploring Pro-Inflammatory Immunological Mediators: Unraveling the Mechanisms of Neuroinflammation in Lysosomal Storage Diseases.Biomedicines. 2023 Apr 1;11(4):1067. doi: 10.3390/biomedicines11041067. Biomedicines. 2023. PMID: 37189685 Free PMC article. Review.
-
Astrocyte-mediated inflammatory responses in traumatic brain injury: mechanisms and potential interventions.Front Immunol. 2025 May 8;16:1584577. doi: 10.3389/fimmu.2025.1584577. eCollection 2025. Front Immunol. 2025. PMID: 40406119 Free PMC article. Review.
References
-
- World Health Organization. 2005. WHO Expert Consultation on Rabies: first report. WHO technical report series no. 931. World Health Organization, Geneva, Switzerland: http://www.who.int/rabies/ExpertConsultationOnRabies.pdf?ua=1 - PubMed
-
- Rupprecht CE. 1996. Rhabdoviruses: rabies virus, Chapter 61. In Baron S. (ed), Medical microbiology, 4th ed. University of Texas Medical Branch at Galveston, Galveston, TX - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

