Treatment for primary postpartum haemorrhage
- PMID: 24523225
- PMCID: PMC6483801
- DOI: 10.1002/14651858.CD003249.pub3
Treatment for primary postpartum haemorrhage
Abstract
Background: Primary postpartum haemorrhage (PPH) is one of the top five causes of maternal mortality in both developed and developing countries.
Objectives: To assess the effectiveness and safety of any intervention used for the treatment of primary PPH.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2013).
Selection criteria: Randomised controlled trials comparing any interventions for the treatment of primary PPH.
Data collection and analysis: We assessed studies for eligibility and quality and extracted data independently. We contacted authors of the included studies to request more information.
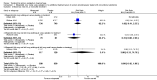
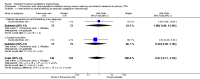
Main results: Ten randomised clinical trials (RCTs) with a total of 4052 participants fulfilled our inclusion criteria and were included in this review.Four RCTs (1881 participants) compared misoprostol with placebo given in addition to conventional uterotonics. Adjunctive use of misoprostol (in the dose of 600 to 1000 mcg) with simultaneous administration of additional uterotonics did not provide additional benefit for our primary outcomes including maternal mortality (risk ratio (RR) 6.16, 95% confidence interval (CI) 0.75 to 50.85), serious maternal morbidity (RR 0.34, 95% CI 0.01 to 8.31), admission to intensive care (RR 0.79, 95% CI 0.30 to 2.11) or hysterectomy (RR 0.93, 95% CI 0.16 to 5.41). Two RCTs (1787 participants) compared 800 mcg sublingual misoprostol versus oxytocin infusion as primary PPH treatment; one trial included women who had received prophylactic uterotonics, and the other did not. Primary outcomes did not differ between the two groups, although women given sublingual misoprostol were more likely to have additional blood loss of at least 1000 mL (RR 2.65, 95% CI 1.04 to 6.75). Misoprostol was associated with a significant increase in vomiting and shivering.Two trials attempted to test the effectiveness of estrogen and tranexamic acid, respectively, but were too small for any meaningful comparisons of pre-specified outcomes.One study compared lower segment compression but was too small to assess impact on primary outcomes.We did not identify any trials evaluating surgical techniques or radiological interventions for women with primary PPH unresponsive to uterotonics and/or haemostatics.
Authors' conclusions: Clinical trials included in the current review were not adequately powered to assess impact on the primary outcome measures. Compared with misoprostol, oxytocin infusion is more effective and causes fewer side effects when used as first-line therapy for the treatment of primary PPH. When used after prophylactic uterotonics, misoprostol and oxytocin infusion worked similarly. The review suggests that among women who received oxytocin for the treatment of primary PPH, adjunctive use of misoprostol confers no added benefit.The role of tranexamic acid and compression methods requires further evaluation. Furthermore, future studies should focus on the best way to treat women who fail to respond to uterotonic therapy.
Conflict of interest statement
Haleema Shakur and Zarko Alfirevic are investigators in the currently ongoing WOMAN trial. Jennifer Blum was a principal investigator in the Blum 2010, Winikoff 2010, Widmer 2010 and Zuberi 2008 trials. Hatem Mousa has received financial support from Novo Nordisk to investigate recombinant activated factor VII (rFVIIa) as a potential treatment for massive postpartum haemorrhage.
Figures
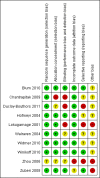
































































































Update of
-
Treatment for primary postpartum haemorrhage.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003249. doi: 10.1002/14651858.CD003249.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2014 Feb 13;(2):CD003249. doi: 10.1002/14651858.CD003249.pub3. PMID: 17253486 Updated.
References
References to studies included in this review
Blum 2010 {published data only}
-
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of post‐partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double‐blind, randomised, non‐inferiority trial. Lancet 2010;375(9710):217‐23. - PubMed
-
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin: results from a randomized noninferiority trial among women receiving prophylactic oxytocin. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S22‐S23.
-
- Dao B, Blum J, Barrera G, Cherine Ramadan M, Dabash R, Darwish E, et al. Side effect profiles for misoprostol and oxytocin in the treatment of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S150.
Chantrapitak 2009 {published data only}
-
- Chantrapitak W, Srijanteok K, Puangsa‐art S. Lower uterine segment compression for management of early postpartum hemorrhage after vaginal delivery at Charoenkrung Pracharak Hospital. Journal of the Medical Association of Thailand 2009;92(5):600‐5. - PubMed
Ducloy‐Bouthors 2011 {published data only}
-
- Ducloy‐Bouthors AS, Duhamel A, Broisin F, Keita H, Fontaine S. Tranexamic acid reduces blood loss in post‐partum haemorrhage by reducing hyperfibrinolysis. British Journal of Anaesthesia 2012;108:ii191.
-
- Ducloy‐Bouthors AS, Duhamel A, Tournoys A, Prado‐Dupont A, Debize G, Peneau E, et al. Post‐partum haemorrhage induced hyperfibrinolysis. Thrombosis Research 2013;131(Suppl 1):S89‐90.
Hofmeyr 2004 {published data only}
-
- Maholwana B, Hofmeyr GJ, Nikodem C, Ferreira S, Singata M, Mangesi L, et al. Misoprostol for treating postpartum haemorrhage. Presented at: 21st Conference on Priorities in Perinatal Care in South Africa. 2002 March 5‐8; Eastern Cape, South Africa.
Lokugamage 2001 {published data only}
-
- Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durban primary postpartum haemorrhage study. Women's Health—into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town, South Africa. 1999:77‐8.
-
- Lokugamage AU, Sullivan KR, Niculescu I, Tigere P, Onyangunga F, Refaey H, et al. A randomized study comparing rectally administered misoprostol versus syntometrine combined with an oxytocin infusion for the cessation of primary post partum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 2001;80(9):835‐9. - PubMed
Walraven 2004 {published data only}
-
- Walraven G, Dampha Y, Bittaye B, Sowe M, Hofmeyr J. Misoprostol in the treatment of postpartum haemorrhage in addition to routine management: a placebo randomised control trial. BJOG: an international journal of obstetrics and gynaecology 2004;111:1014‐7. - PubMed
Widmer 2010 {published data only}
-
- Villar J. Misoprostol to treat postpartum hemorrhage (PPH): a randomized controlled trial (Argentina, Egypt, South Africa, Thailand and Viet Nam). Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 21 March 2006).
-
- Widmer M, Blum J, Hofmeyr GJ, Carroli G, Abdel‐Aleem H, Lumbiganon P, et al. Misoprostol as an adjunct to standard uterotonics for treatment of post‐partum haemorrhage: a multicentre, double‐blind randomised trial. Lancet 2010;375(9728):1808‐13. - PubMed
Winikoff 2010 {published data only}
-
- Winikoff B. Misoprostol for the treatment of postpartum hemorrhage. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
-
- Winikoff B, Dabash R, Durocher J, Darwish E, Ngoc NTN, Leon W, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin: results from a randomized, non‐inferiority trial among women not exposed to oxytocin during labor. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S59.
-
- Winikoff B, Dabash R, Durocher J, Darwish E, Nguyen TN, Leon W, et al. Treatment of post‐partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labour: a double‐blind, randomised, non‐inferiority trial. Lancet 2010;375(9710):210‐6. - PubMed
Zhou 2006 {published data only}
-
- Zhou M, Yang CY, Zhao Y, Li P. Clinical value of adjuvant therapy with estrogen for postpartum hemorrhage. Journal of Southern Medical University 2006;26(6):865‐6. - PubMed
References to studies excluded from this review
Deneux‐Tharaux 2010 {published data only}
-
- Deneux‐Tharaux C, Dupont C, Colin C, Rabilloud M, Touzet S, Lansac J, et al. Multifaceted intervention to decrease the rate of severe postpartum haemorrhage: the PITHAGORE6 cluster‐randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2010;117(10):1278‐87. - PMC - PubMed
Khalil 2011 {published data only}
-
- Khalil MI, Al‐Dohami H, Aldahish MM. A method to improve the effectiveness of the Bakri balloon for management of postpartum haemorrhage at caesarean. International Journal of Gynaecology and Obstetrics 2011;115(2):198‐200. - PubMed
Khireddine 2012 {published data only}
-
- Khireddine I, Le C, Dupont C, Rudigoz R, Bouvier‐Colle M, Deneux‐Tharaux C. Induction of labor and risk of postpartum hemorrhage in low risk parturient women. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):101.
Magwali 2012 {published data only}
-
- Magwali TL, Butrick E, Mambo V, Ayadi A, Lippman S, Bergel E, et al. Non‐pneumatic anti‐shock garment (NASG) for obstetric hemorrhage:Harare, Zimbabwe. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S410.
Soltan 2009 {published data only}
-
- Soltan MH, Faragallah MF, Mosabah MH, Al‐Adawy AR. External aortic compression device: the first aid for postpartum hemorrhage control. Journal of Obstetrics and Gynaecology Research 2009;35(3):453‐8. - PubMed
Soltan 2010 {published data only}
-
- Soltan MH, Imam HH, Zahran KA, Atallah SM. Assessing changes in flow velocimetry and clinical outcome following use of an external aortic compression device in women with postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2010;110(3):257‐61. - PubMed
Takagi 1976 {published data only}
-
- Takagi S, Yoshida T, Togo Y, Tochigi H, Abe M, Sakata H, et al. The effects of intramyometrial injection of prostaglandin F2alpha on severe post‐partum hemorrhage. Prostaglandins 1976;12(4):565‐79. - PubMed
References to studies awaiting assessment
Lavigne‐Lissalde 2013 {published data only}
-
- Gris JC. rhuFVIIa in post‐partum hemorrhage. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
-
- Lavigne‐Lissalde G, Aya G, Mercier F, Roger‐Christophe S, Chauleur C, Morau E, et al. rhuFVIIa in women with a refractory primary postpartum haemorrhage: an international, multicenter, randomised, opened, controlled trial. Thrombosis Research 2013;131(Suppl 1):S74.
References to ongoing studies
Collins 2013 {published data only}
-
- Collins P. Fibrinogen concentrate versus placebo for treatment of postpartum haemorrhage: A multicentre, prospective, double blind randomised control trial. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 2013).
Miller 2008 {published data only}
-
- Miller S. Non‐pneumatic anti‐shock garment for obstetrical hemorrhage: Zambia and Zimbabwe. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Mirzazada 2011 {published data only}
-
- Mirzazada S. Misoprostol for the treatment of postpartum haemorrhage (PPH) following self‐administration of misoprostol prophylaxis in home deliveries. ClinicalTrials.gov (accessed 21 May 2013).
Shakur 2010 {published data only}
-
- Gulmezoglu M, Alfirevic Z, Elbourne D, Roberts I. Tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind, placebo controlled trial (woman trial—Protocol Number ISRCTN76912190). International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S500. - PMC - PubMed
Wikkelsoe 2012 {published data only}
Additional references
Abdel‐Aleem 2001
-
- Abdel‐Aleem H, El‐Nashar I, Abdel‐Aleem A. Management of severe postpartum hemorrhage with misoprostol. International Journal of Gynecology & Obstetrics. 2001;72:75‐6. - PubMed
Abdel‐Aleem 2003
-
- Abdel‐Aleem H, Villar J, Gulmezoglu AM, Mostafa SA, Youssef AA, Shokry M, et al. The pharmacokinetics of the prostaglandin E1 analogue misoprostol in plasma and colostrum after postpartum oral administration. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;108:25‐8. - PubMed
AbdRabbo 1994
-
- AbdRabbo SA. Stepwise uterine devascularization: a novel technique for the management of uncontrollable postpartum hemorrhage with preservation of the uterus. American Journal of Obstetrics and Gynecology 1994;171:694‐700. - PubMed
Abou El Senoun 2011
-
- Abou El Senoun G, Singh M, Mousa HA, Alfirevic Z. Update on the new modalities on the prevention and management of postpartum haemorrhage. Fetal and Maternal Medicine Review 2011;22(4):247‐64.
Abou Zahr 1991
-
- Abou Zahr C, Royston E. Global Mortality: Global Factbook. Geneva: World Health Organization, 1991.
ACOG 1998
-
- ACOG Technical Bulletin. American College of Obstetricians and Gynecologists educational bulletin. Postpartum hemorrhage, Number 243. International Journal of Gynecology & Obstetrics 1998;61(1):79‐86. - PubMed
Adekanmi 2001
-
- Adekanmi OA, Purmessur S, Edwards G, Barrington JW. Intrauterine misoprostol for the treatment of severe recurrent atonic secondary postpartum haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2001;108:541‐2. - PubMed
Aisaka 1988
-
- Aisaka K, Ando S, Kokuho K, Tawada T, Kaneda S, Yoshimatsu J, et al. Effects of obesity and weight gain during pregnancy on obstetrical factors. Acta Obstetrica et Gynaecologica Japonica 1988;63:1851‐8. - PubMed
Akhter 2005
-
- Akhter S, Begum MR, Kabir J. Condom hydrostatic tamponade for massive postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2005;90:134–5. - PubMed
Alamia 1999
-
- Alamia V Jr, Meyer BA. Peripartum hemorrhage. Obstetrics and Gynecology Clinics of North America 1999;26(2):385‐98. - PubMed
Alexander 2002
Alfirevic 2006
Alfirevic 2007
-
- Alfirevic Z, Elbourne D, Pavord S, Bolte A, Geijn H, Mercier F, et al. Use of recombinant activated factorVII in primary postpartum hemorrhage: the Northern European registry 2000‐2004. Obstetrics and Gynecology 2007;110(6):1270‐8. - PubMed
Andolina 2003
-
- Andolina KL, Tolosa JE, Monzo JM, Roberts NS, Daly S. Oral misoprostol is rapidly absorbed in postpartum women at term. Journal of Maternal‐Fetal & Neonatal Medicine 2003;14:229‐32. - PubMed
Arulkumaran 2007
-
- Arulkumaran S, Walker JJ, Watkinson AF, Nicholson T, Kessel D, Patel J. The Role of Emergency and Elective Interventional Radiology in Postpartum Haemorrhage: Good Practice No 6. London: RCOG, 2007.
As 1996
-
- As AK, Hagen P, Webb JB. Tranexamic acid in the management of postpartum haemorrhage. British Journal of Obstetrics and Gynaecology 1996;103(12):1250‐1. - PubMed
B‐Lynch 1997
-
- B‐Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B‐Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. British Journal of Obstetrics and Gynaecology 1997;104(3):372‐5. - PubMed
Bakri 1999
-
- Bakri YN. Balloon device for control of obstetrical bleeding. European Journal of Obstetrics & Gynecology, and Reproductive Biology 1999;86:S84.
Baskett 2000
Begley 2011
Beischer 1986
-
- Beischer NA, Mackay EV. Obstetrics and the Newborn. Eastbourne: Bailliere Tindall, 1986.
Bergstrom 1962
-
- Bergstrom S, Ryhag ER, Samuelson B, Sjovall J. The structure of prostaglandin E, F1, F2. Acta Chemica Scandinavica 1962;16:501‐2.
Bonnar 2000
-
- Bonnar J. Massive obstetric haemorrhage. Baillieres Best Practice and Research in Clinical Obstetrics and Gynaecology 2000;14(1):1‐18. - PubMed
Burchell 1980
-
- Burchell RC. Postpartum haemorrhage. In: Quilligan ES editor(s). Current Therapy in Obstetrics and Gynaecology. Philadelphia: WB Saunders, 1980.
Cantwell 2011
-
- Cantwell R, Clutton‐Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG: an international journal of obstetrics and gynaecology 2011;118(Suppl 1):1‐203. - PubMed
Carroli 2008
-
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum hemorrhage: a systematic review. Best Practice and Research. Clinical Obstetrics and Gynaecology 2008;22(6):999‐1020. - PubMed
Chan 1997
-
- Chan C, Razvi K, Tham KF, Arulkumaran S. The use of a Sengstaken‐Blakemore tube to control post‐partum hemorrhage. International Journal of Gynaecology and Obstetrics 1997;58:251–2. - PubMed
Cho 2000
-
- Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstetrics & Gynecology 2000;96(1):129‐31. - PubMed
Clark 1985
-
- Clark SL, Phelan JP, Yeh SY, Bruce SR, Paul RH. Hypogastric artery ligation for obstetric hemorrhage. Obstetrics & Gynecology 1985;66(3):353‐6. - PubMed
Combs 1991
-
- Combs CA, Murphy EL, Laros RK. Factors associated with postpartum haemorrhage with vaginal birth. Obstetrics & Gynecology 1991;77:69‐76. - PubMed
Cotter 2001
CRASH‐2 trial collaborators 2010
-
- CRASH‐2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet 2010;376(9734):23‐32. - PubMed
Cunningham 1993
-
- Cunningham FG, MacDonald PC, Gant NF, Leveno KJ, Gilstrap LC. Abnormalities of the third stage of labor. Williams Obstetrics. 19th Edition. Norwalk, CT: Appleton & Lange, 1993.
Cutler 1971
Danielsson 1999
-
- Danielsson KG, Marions L, Rodriguez A, Spur BW, Wong PY, Bygdeman M. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstetrics & Gynecology 1999;93:275‐80. - PubMed
Davis 1935
-
- Davis ME, Adher FL, Rogers G, Kharasch MS, Legault RR. A new active principle in ergot and its effects on uterine motility. American Journal of Obstetrics and Gynecology 1935;29:155‐67. - PubMed
De Loor 1996
-
- Loor JA, Dam PA. Foley catheters for uncontrollable obstetric or gynecologic hemorrhage. Obstetrics & Gynecology 1996;88:737–8. - PubMed
Dodd 2010
Doumouchtsis 2007
-
- Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstetrical and Gynecological Survey 2007;62(8):540‐7. - PubMed
Drife 1997
-
- Drife J. Management of primary postpartum haemorrhage. British Journal of Obstetrics and Gynaecology 1997;104:275‐7. - PubMed
Du Vigneaud 1953
-
- Du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG, Gordon S. The synthesis of an octapeptide with the hormonal activity of oxytocin. Journal of the American Chemical Society 1953;75:4879‐80.
Dudley 1935
Eastman 1950
-
- Eastman NJ. Anomalies of the third stage of labor. William's Obstetrics. 10th Edition. New York: Appleton‐Century‐Crofts, 1950:917‐9.
Ferrer 2009
Franchini 2010
-
- Franchini M, Franchi M, Bergamini V, Montagnana M, Salvagno GL, Targher G, et al. The use of recombinant activated FVII in postpartum haemorrhage. Clinical Obstetrics and Gynecology 2010;53:219‐27. - PubMed
Gando 2006
-
- Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Critical Care Medicine 2006;34(3):625‐31. - PubMed
Georgiou 2009
-
- Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG: an international journal of obstetrics and gynaecology 2009;116:748‐57. - PubMed
Gilbert 1987
-
- Gilbert L, Porter W, Brown VA. Postpartum haemorrhage: a continuing problem. British Journal of Obstetrics and Gynaecology 1987;94:67‐71. - PubMed
Gyte 1992
-
- Gyte G. The significance of blood loss at delivery. MIDIRS Midwifery Digest 1992;2(1):88‐92.
Gülmezoglu 2001
-
- Gülmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358(9283):689‐95. - PubMed
Haggerty 1996
-
- Haggerty J. Antishock garment. http://www.sti.nasa.gov/tto/spinoff1996/28. (accessed 20 February 2008).
Hall 1985
-
- Hall MH, Halliwell R, Carr‐Hill R. Concomitant and repeated happenings of complications of the third stage of labour. British Journal of Obstetrics and Gynaecology 1985;92:732‐8. - PubMed
Hayman 2002
-
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstetrics & Gynecology 2002;99(3):502‐6. - PubMed
Hensleigh 2002
-
- Hensleigh PA. Anti‐shock garment provides resuscitation and haemostasis for obstetric haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2002;109(12):1377‐84. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2005
-
- Hofmeyr GJ, Walraven G, Gulmezoglu AM, Maholwana B, Alfirevic Z, Villar J. Misoprostol to treat postpartum haemorrhage: a systematic review. BJOG: an international journal of obstetrics and gynaecology 2005;112:547‐53. - PubMed
Hofmeyr 2008
-
- Hofmeyr GJ, Gülmezoglu AM. Misoprostol for the prevention and treatment of postpartum haemorrhage. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22(6):1025‐41. - PubMed
Hunter 1992
-
- Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long acting oxytocin analogue in the postpartum uterus. Clinical Pharmacology and Therapeutics 1992;52:60‐7. - PubMed
Johanson 2001
-
- Johanson R, Kumar M, Obhrai M, Young P. Management of massive postpartum haemorrhage: use of a hydrostatic balloon catheter to avoid laparotomy. BJOG: an international journal of obstetrics and gynaecology 2001;108(4):420‐2. - PubMed
Jouppila 1995
-
- Jouppila P. Postpartum haemorrhage. Current Opinion in Obstetrics and Gynecology 1995;7:446‐50. - PubMed
Khan 2003
-
- Khan RU, El‐Refaey H. Pharmacokinetics and adverse‐effect profile of rectally administered misoprostol in the third stage of labour. Obstetrics & Gynecology 2003;101:968‐74. - PubMed
Khan 2006
-
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066‐74. - PubMed
Knight 2009
Liabsuetrakul 2007
Maier 1993
-
- Maier RC. Control of postpartum haemorrhage with uterine packing. American Journal of Obstetrics and Gynecology 1993;169:317‐23. - PubMed
Marasinghe 2011
-
- Marasinghe JP, Condous G, Seneviratne HR, Marasinghe U. Modified anchored B‐Lynch uterine compression suture for post partum bleeding with uterine atony. Acta Obstetricia et Gynecologica Scandinavica 2011;90:280–3. - PubMed
McDonald 2004
McDonald 2013
Miller 2009
-
- Miller S, Ojengbede O, Turan JM, Morhason‐Bello IO, Martin HB, Nsima D. A comparative study of the non‐pneumatic anti‐shock garment for the treatment of obstetric hemorrhage in Nigeria. International Journal of Gynecology & Obstetrics 2009;107(2):121‐5. - PubMed
Miller 2010
Mobeen 2011
-
- Mobeen N, Durocher J, Zuberi N, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353–61. - PMC - PubMed
Moir 1932
Moscardo 2001
-
- Moscardo F, Perez F, Rubia J, Balerdi B, Lorenzo JI, Senent ML, et al. Successful treatment of severe intra‐abdominal bleeding associated with disseminated intravascular coagulation using recombinant activated factor VII. British Journal of Haematology 2001;114:174‐6. - PubMed
Mousa 2001
-
- Mousa HA, Walkinshaw S. Major postpartum haemorrhage. Current Opinion in Obstetrics and Gynecology 2001;13(6):595‐603. - PubMed
Mousa 2002
-
- Mousa H, Alfirevic Z. Major postpartum hemorrhage: survey of maternity units in the United Kingdom. Acta Obstetricia et Gynecologica Scandinavica 2002;81(8):727‐30. - PubMed
Mousa 2008
-
- Mousa HA, Cording V, Alfirevic Z. Risk factors and interventions associated with major primary postpartum hemorrhage unresponsive to first‐line conventional therapy. Acta Obstetricia et Gynecologica Scandinavica 2008;87(6):652‐61. - PubMed
Nardin 2011
Novikova 2010
O'Brien 1998
-
- O'Brien P, El‐Refaey H, Gordon A, Geary M, Rodeck CH. Rectally administered misoprostol for the treatment of postpartum haemorrhage unresponsive to oxytocin and ergometrine: a descriptive study. Obstetrics & Gynecology 1998;92:212‐4. - PubMed
Oboro 2003
-
- Oboro VO, Tabowei TO, Bosah JO. Intrauterine misoprostol for refractory postpartum haemorrhage. International Journal of Gynecology & Obstetrics 2003;80:67‐8. - PubMed
Ochoa 2002
-
- Ochoa M, Allaire AD, Stitely ML. Pyometria after hemostatic square suture technique. Obstetrics & Gynecology 2002;99(3):506‐9. - PubMed
Ojengbede 2011
-
- Ojengbede OA, Morhason‐Bello IO, Galadanci H, Meyer C, Nsima D, Camlin C, et al. Assessing the role of the non‐pneumatic anti‐shock garment in reducing mortality from postpartum hemorrhage in Nigeria. Gynecologic and Obstetric Investigation 2011;71(1):66‐72. - PubMed
Oladapo 2012a
Oladapo 2012b
Oleen 1990
-
- Oleen MA, Mariano JP. Controlling refractory atonic postpartum hemorrhage with hemabate sterile solution. American Journal of Obstetrics and Gynecology 1990;162(1):205‐8. - PubMed
Ouahba 2007
-
- Ouahba J, Piketty M, Huel C, Azarian M, Feraud O, Luton D, et al. Uterine compression sutures for postpartum bleeding with uterine atony. BJOG: an international journal of obstetrics and gynaecology 2007;114(5):619‐22. - PubMed
Peitsidis 2011
-
- Peitsidis P, Kadir RA. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opinion on Pharmacotherapy 2011;12(4):503‐16. - PubMed
Penninx 2010
-
- Penninx JP, Pasmans HL, Oei SG. Arterial balloon occlusion of the internal iliac arteries for treatment of life‐threatening massive postpartum haemorrhage: a series of 15 consecutive cases. European Journal of Obstetrics & Gynecology, and Reproductive Biology 2010;148(2):131‐4. - PubMed
Pereira 2005
-
- Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstetrics and Gynecology 2005;106(3):569‐72. - PubMed
Porro 1876
-
- Porro E. Della amputazione utero‐ovarica come complemento di taglio cesareo. Milan 1876.
Poujade 2011
-
- Poujade O, Grossetti A, Mougel L, Ceccaldi P, Ducarme G, Luton D. Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2011;118:433–9. - PubMed
Prendiville 1989
-
- Prendiville WJ, Elbourne DR. Care during the third stage of labour.. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective care in pregnancy and childbirth. Oxford: Oxford University Press,, 1989:1145–69.
Pritchard 1962
-
- Pritchard JA, Baldwin RM, Dickey JC, Wiggins KM. Blood volume changes in pregnancy and puerperium, II: red blood cell loss and changes in apparent blood volume during and following vaginal delivery, cesarean section, and cesarean section plus total hysterectomy. American Journal of Obstetrics and Gynecology 1962;84:1271‐82.
Rath 2009
-
- Rath W. Prevention of postpartum haemorrhage with the oxytocin analogue carbetocin. European Journal of Obstetrics & Gynecology, and Reproductive Biology 2009;147(1):15‐20. - PubMed
Rathat 2011
-
- Rathat G, Do Trinh P, Mercier G, Reyftmann L, Dechanet C, Boulot P, et al. Synechia after uterine compression sutures. Fertility and Sterility 2011;95(1):405‐9. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ripley 1999
-
- Ripley DL. Uterine emergencies. Atony, inversion, and rupture. Obstetrics and Gynecology Clinics of North America 1999;26(3):419‐34. - PubMed
Sloan 2010
Stafford 2008
-
- Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. American Journal of Obstetrics and Gynecology 2008;199(5):e511–e517. - PubMed
Stearns 1808
-
- Stearns J. Account of the pulvis parturiens, a remedy for quickening childbirth. Medical Repository of New York 1808;11:308‐9.
Stearns 1822
-
- Steans J. Observations on the secale cornutum or ergot, with directions for its use in parturition. Medical Research 1822;5:90.
Stoll 1935
-
- Stoll A, Burckhardt E. L'ergobasine, nouvel alkaloide de l'ergot de seigle, solubile dans l'eau. Bulletin of Pharmaceutical Sciences 1935;42:257‐66.
Stones 1993
-
- Stones RW, Paterson CM, Saunders NSTG. Risk factors for major obstetric haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:15‐8. - PubMed
Su 2007
Su 2012
Suzuki 2012
-
- Suzuki S, Hiraizumi Y, Miyake H. Risk factors for postpartum hemorrhage requiring transfusion in cesarean deliveries for Japanese twins: comparison with those for singletons. Archives of Gynecology and Obstetrics 2012;286(6):1363‐7. - PubMed
Tang 2002
-
- Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Human Reproduction 2002;17:332‐6. - PubMed
Thompson 1935
-
- Thompson MR. The active constituents of ergot. A pharmacological and chemical study. Journal of the American Pharmaceutical Association 1935;24:185‐96.
Tseng 2011
-
- Tseng JJ, Ho JY, Wen MC, Hwang JI. Uterine necrosis associated with acute suppurative myometritis after angiographic selective embolization for refractory postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2011;204(6):e4‐e6. - PubMed
Tsu 1993
-
- Tsu VD. Postpartum haemorrhage in Zimbabwe: a risk factor analysis. British Journal of Obstetrics and Gynaecology 1993;100:327‐33. - PubMed
Tunçalp 2012
Vahedi 1995
-
- Vahedi M, Ayuyao A, Parsa M, Freeman H. Pneumatic antishock garment‐associated compartment syndrome in uninjured lower extremities. Journal of Trauma 1995;384(4):616‐8. - PubMed
Wetta 2013
WHO 2010
-
- WHO, UNICEF, UNFPA. The World Bank World Health Organization (WHO) Press. Trends in Maternal Mortality: 1990 to 2008: Estimates Developed by WHO, UNICEF, UNFPA and The World Bank. Geneva: WHO, 2010.
Zheng 2011
-
- Zheng J, Xiong X, Ma Q, Zhang X, Li M. A new uterine compression suture for postpartum haemorrhage with atony. BJOG: an international journal of obstetrics and gynaecology 2011;118:370–4. - PubMed
Zieman 1997
-
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. - PubMed
References to other published versions of this review
Hofmeyr 2009
Mousa 2003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

