Astroglial-derived periostin promotes axonal regeneration after spinal cord injury
- PMID: 24523534
- PMCID: PMC6802751
- DOI: 10.1523/JNEUROSCI.2947-13.2014
Astroglial-derived periostin promotes axonal regeneration after spinal cord injury
Abstract
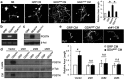
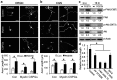
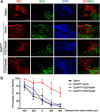
Traumatic spinal cord injury (SCI) results in a cascade of tissue responses leading to cell death, axonal degeneration, and glial scar formation, exacerbating the already hostile environment and further inhibiting axon regeneration. Overcoming these inhibitory cues and promoting axonal regeneration is one of the primary targets in developing a cure for SCI. Previously, we demonstrated that transplantation of bone morphogenetic protein (BMP)-induced astrocytes derived from embryonic glial-restricted precursors (GDAs(BMP)) promotes extensive axonal growth and motor function recovery in a rodent spinal cord injury model. Here, we identify periostin (POSTN), a secreted protein, as a key component of GDA(BMP)-induced axonal regeneration. POSTN is highly expressed by GDAs(BMP) and the perturbation of POSTN expression by shRNA diminished GDA(BMP)-induced neurite extension in vitro. We also found that recombinant POSTN is sufficient to overcome the inhibitory effect of scar-associated molecules and promote neurite extension in vitro by signaling through focal adhesion kinase and Akt. Furthermore, transplantation of POSTN-deficient GDAs(BMP) into the injured rat spinal cord resulted in compromised axonal regeneration, indicating that POSTN plays an essential role in GDA(BMP)-mediated axonal regeneration. This finding reveals not only one of the major mechanisms underlying GDA(BMP)-dependent recovery from SCI, but also the potential of POSTN as a therapeutic agent for traumatic injury of the CNS.
Keywords: astrocyte; cell therapy; glial precursor; neurite outgrowth; spinal cord; support cell.
Figures

References
-
- Chen J, Joon Lee H, Jakovcevski I, Shah R, Bhagat N, Loers G, Liu HY, Meiners S, Taschenberger G, Kügler S, Irintchev A, Schachner M. The extracellular matrix glycoprotein tenascin-C is beneficial for spinal cord regeneration. Mol Ther. 2010;18:1769–1777. doi: 10.1038/mt.2010.133. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
