The thalamostriatal system in normal and diseased states
- PMID: 24523677
- PMCID: PMC3906602
- DOI: 10.3389/fnsys.2014.00005
The thalamostriatal system in normal and diseased states
Abstract
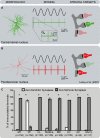
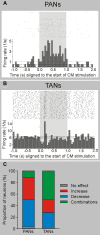
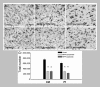
Because of our limited knowledge of the functional role of the thalamostriatal system, this massive network is often ignored in models of the pathophysiology of brain disorders of basal ganglia origin, such as Parkinson's disease (PD). However, over the past decade, significant advances have led to a deeper understanding of the anatomical, electrophysiological, behavioral and pathological aspects of the thalamostriatal system. The cloning of the vesicular glutamate transporters 1 and 2 (vGluT1 and vGluT2) has provided powerful tools to differentiate thalamostriatal from corticostriatal glutamatergic terminals, allowing us to carry out comparative studies of the synaptology and plasticity of these two systems in normal and pathological conditions. Findings from these studies have led to the recognition of two thalamostriatal systems, based on their differential origin from the caudal intralaminar nuclear group, the center median/parafascicular (CM/Pf) complex, or other thalamic nuclei. The recent use of optogenetic methods supports this model of the organization of the thalamostriatal systems, showing differences in functionality and glutamate receptor localization at thalamostriatal synapses from Pf and other thalamic nuclei. At the functional level, evidence largely gathered from thalamic recordings in awake monkeys strongly suggests that the thalamostriatal system from the CM/Pf is involved in regulating alertness and switching behaviors. Importantly, there is evidence that the caudal intralaminar nuclei and their axonal projections to the striatum partly degenerate in PD and that CM/Pf deep brain stimulation (DBS) may be therapeutically useful in several movement disorders.
Keywords: Parkinson’s disease; Tourette’s syndrome; attention; glutamate; intralaminar nuclei; striatum; thalamus; vesicular glutamate transporter.
Figures



Similar articles
-
The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states.Brain Res Bull. 2009 Feb 16;78(2-3):60-8. doi: 10.1016/j.brainresbull.2008.08.015. Epub 2008 Sep 19. Brain Res Bull. 2009. PMID: 18805468 Free PMC article. Review.
-
Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats.J Comp Neurol. 2006 Nov 10;499(2):231-43. doi: 10.1002/cne.21099. J Comp Neurol. 2006. PMID: 16977615 Free PMC article.
-
Thalamic degeneration in MPTP-treated Parkinsonian monkeys: impact upon glutamatergic innervation of striatal cholinergic interneurons.Brain Struct Funct. 2019 Dec;224(9):3321-3338. doi: 10.1007/s00429-019-01967-w. Epub 2019 Nov 2. Brain Struct Funct. 2019. PMID: 31679085 Free PMC article.
-
Expression of vesicular glutamate transporters 1 and 2 in the cells of origin of the rat thalamostriatal pathway.J Chem Neuroanat. 2008 Jan;35(1):101-7. doi: 10.1016/j.jchemneu.2007.08.001. Epub 2007 Aug 8. J Chem Neuroanat. 2008. PMID: 17826944
-
The thalamostriatal system: a highly specific network of the basal ganglia circuitry.Trends Neurosci. 2004 Sep;27(9):520-7. doi: 10.1016/j.tins.2004.07.004. Trends Neurosci. 2004. PMID: 15331233 Review.
Cited by
-
Combinatorial Developmental Controls on Striatonigral Circuits.Cell Rep. 2020 Jun 16;31(11):107778. doi: 10.1016/j.celrep.2020.107778. Cell Rep. 2020. PMID: 32553154 Free PMC article.
-
Cholinergic Transmission at Muscarinic Synapses in the Striatum Is Driven Equally by Cortical and Thalamic Inputs.Cell Rep. 2019 Jul 23;28(4):1003-1014.e3. doi: 10.1016/j.celrep.2019.06.077. Cell Rep. 2019. PMID: 31340139 Free PMC article.
-
Maladaptive Synaptic Plasticity in L-DOPA-Induced Dyskinesia.Front Neural Circuits. 2016 Dec 20;10:105. doi: 10.3389/fncir.2016.00105. eCollection 2016. Front Neural Circuits. 2016. PMID: 28066191 Free PMC article. Review.
-
Parkinson's Disease: A Thalamostriatal Rebalancing Act?Neuron. 2016 Feb 17;89(4):675-7. doi: 10.1016/j.neuron.2016.02.008. Neuron. 2016. PMID: 26889806 Free PMC article.
-
Study on Lesion Assessment of Cerebello-Thalamo-Cortical Network in Wilson's Disease with Diffusion Tensor Imaging.Neural Plast. 2017;2017:7323121. doi: 10.1155/2017/7323121. Epub 2017 Jul 11. Neural Plast. 2017. PMID: 28781902 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

