Ablation of the proapoptotic genes CHOP or Ask1 does not prevent or delay loss of visual function in a P23H transgenic mouse model of retinitis pigmentosa
- PMID: 24523853
- PMCID: PMC3921110
- DOI: 10.1371/journal.pone.0083871
Ablation of the proapoptotic genes CHOP or Ask1 does not prevent or delay loss of visual function in a P23H transgenic mouse model of retinitis pigmentosa
Abstract
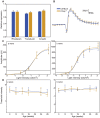
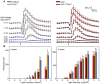
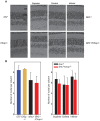
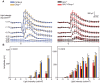
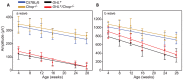
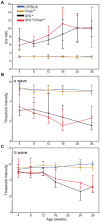
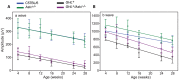
The P23H mutation in rhodopsin (Rho(P23H)) is a prevalent cause of autosomal dominant retinitis pigmentosa. We examined the role of the ER stress proteins, Chop and Ask1, in regulating the death of rod photoreceptors in a mouse line harboring the Rho(P23H) rhodopsin transgene (GHL(+)). We used knockout mice models to determine whether Chop and Ask1 regulate rod survival or retinal degeneration. Electrophysiological recordings showed similar retinal responses and sensitivities for GHL(+), GHL(+)/Chop(-/-) and GHL(+)/Ask1(-/-) animals between 4-28 weeks, by which time all three mouse lines exhibited severe loss of retinal function. Histologically, ablation of Chop and Ask1 did not rescue photoreceptor loss in young animals. However, in older mice, a regional protective effect was observed in the central retina of GHL(+)/Chop(-/-) and GHL(+)/Ask1(-/-), a region that was severely degenerated in GHL(+) mice. Our results show that in the presence of the Rho(P23H) transgene, the rate of decline in retinal sensitivity is similar in Chop or Ask1 ablated and wild-type retinas, suggesting that these proteins do not play a major role during the acute phase of photoreceptor loss in GHL(+) mice. Instead they may be involved in regulating secondary pathological responses such as inflammation that are upregulated during later stages of disease progression.
Conflict of interest statement
Figures

References
-
- Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368: 1795–1809. - PubMed
-
- Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH (1984) Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol 97: 357–365. - PubMed
-
- Grondahl J (1987) Estimation of prognosis and prevalence of retinitis pigmentosa and Usher syndrome in Norway. Clin Genet 31: 255–264. - PubMed
-
- Papermaster DS, Dreyer WJ (1974) Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry 13: 2438–2444. - PubMed
-
- Mendes HF, van der Spuy J, Chapple JP, Cheetham ME (2005) Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med 11: 177–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

