Regulation of CDK9 activity by phosphorylation and dephosphorylation
- PMID: 24524087
- PMCID: PMC3913462
- DOI: 10.1155/2014/964964
Regulation of CDK9 activity by phosphorylation and dephosphorylation
Abstract
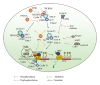
HIV-1 transcription is regulated by CDK9/cyclin T1, which, unlike a typical cell cycle-dependent kinase, is regulated by associating with 7SK small nuclear ribonuclear protein complex (snRNP). While the protein components of this complex are well studied, the mechanism of the complex formation is still not fully understood. The association of CDK9/cyclin T1 with 7SK snRNP is, in part, regulated by a reversible CDK9 phosphorylation. Here, we present a comprehensive review of the kinases and phosphatases involved in CDK9 phosphorylation and discuss their role in regulation of HIV-1 replication and potential for being targeted for drug development. We propose a novel pathway of HIV-1 transcription regulation via CDK9 Ser-90 phosphorylation by CDK2 and CDK9 Ser-175 dephosphorylation by protein phosphatase-1.
Figures

References
-
- Lafeuillade A, Stevenson M. The search for a cure for persistent HIV reservoirs. AIDS Reviews. 2011;13(2):63–66. - PubMed
-
- Watanabe Y, Fujimoto H, Watanabe T, et al. Modulation of TFIIH-associated kinase activity by complex formation and its relationship with CTD phosphorylation of RNA polymerase II. Genes to Cells. 2000;5(5):407–423. - PubMed
-
- Ping Y-H, Rana TM. DSIF and NELF interact with RNA polymerase II elongation complex and HIV-1 Tat stimulates P-TEFb-mediated phosphorylation of RNA polymerase II and DSIF during transcription elongation. The Journal of Biological Chemistry. 2001;276(16):12951–12958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

