Fixed airflow obstruction in asthma: a descriptive study of patient profiles and effect on treatment responses
- PMID: 24524222
- PMCID: PMC4162502
- DOI: 10.3109/02770903.2014.895012
Fixed airflow obstruction in asthma: a descriptive study of patient profiles and effect on treatment responses
Abstract
Background: The role of fixed airflow obstruction (FAO) in asthma is unclear.
Objective: To assess the relationship between FAO and clinical features of asthma and the effect of FAO on treatment response.
Methods: Post hoc descriptive analysis of data stratified by FAO category (screening post-albuterol FEV1/FVC <lower limit of normal [LLN] [FAO+] or ≥LLN [FAO-]) from two 12-week, randomized, placebo-controlled studies of budesonide/formoterol or the monocomponents in mild-moderate (study I; aged ≥6 years; NCT00651651; placebo run-in) or moderate-severe (study II; ≥12 years; NCT00652002; budesonide run-in) asthma patients.
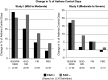
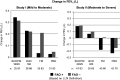
Results: At baseline, FAO+ versus FAO- patients were more likely male and had longer asthma duration and worse pulmonary function. During the treatment period, lung function and asthma control measures with placebo were generally worse in FAO+ versus FAO- patients. Budesonide was effective on most end points in both FAO+ and FAO- patients. In contrast to FAO- patients, FAO+ patients were unresponsive to formoterol monotherapy in both study populations. Consistently greater improvements in most end points (including worsening of asthma as predefined by specific lung function parameters or clinical symptoms) were observed moving from formoterol to budesonide to budesonide/formoterol in both FAO+ and FAO- patients, with generally greater than additive effects on lung function with budesonide/formoterol in FAO+ patients.
Conclusions: FAO+ patients tended to be more impaired and at greater risk for an asthma event versus FAO- patients. While FAO+ patients were non-responsive to formoterol monotherapy, they retained responsiveness to budesonide and had the greatest lung function and control responses to budesonide/formoterol that were similar to or greater than responses of FAO- patients.
Keywords: Airflow limitation; airway inflammation; budesonide; combination therapy; formoterol; inhaled corticosteroid; lung function.
Figures

References
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2012 update. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_March13.pdf [last accessed 19 Nov 2013]
-
- Lee JH, Haselkorn T, Borish L, Rasouliyan L, Chipps BE, Wenzel SE. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: insights from the TENOR study. Chest. 2007;132:1882–1889. - PubMed
-
- Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–2015. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
