Cerebral white and gray matter injury in newborns: new insights into pathophysiology and management
- PMID: 24524444
- PMCID: PMC3947650
- DOI: 10.1016/j.clp.2013.11.001
Cerebral white and gray matter injury in newborns: new insights into pathophysiology and management
Abstract
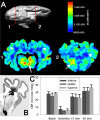
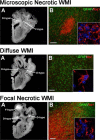
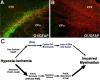
Increasing numbers of preterm neonates survive with motor and cognitive disabilities related to less destructive forms of cerebral injury that still result in reduced cerebral growth. White matter injury results in myelination disturbances related to aberrant responses to death of pre-myelinating oligodendrocytes (preOLs). PreOLs are rapidly regenerated but fail to mature to myelinating cells. Although immature projection neurons are more resistant to hypoxia-ischemia than preOLs, they display widespread disturbances in dendritic arbor maturation, which provides an explanation for impaired cerebral growth. Thus, large numbers of cells fail to fully mature during a critical window in development of neural circuitry. These recently recognized forms of cerebral gray and white matter dysmaturation suggest new therapeutic directions centered on reversal of the processes that promote dysmaturation.
Keywords: Cerebral; Gray matter; Management; Newborns; Pathophysiology; White matter.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Synnes AR, Anson S, Arkesteijn A, et al. School Entry Age Outcomes for Infants with Birth Weight </=800 Grams. J Pediatr. 2010 Jul 31;157(6):989–94. - PubMed
-
- Wilson-Costello D, Fridedman H, Minich N, Fanaroff A, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115(4):997–1003. - PubMed
-
- Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004 Nov 17;292(19):2357–65. - PubMed
-
- Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000 Oct 18;284(15):1939–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

